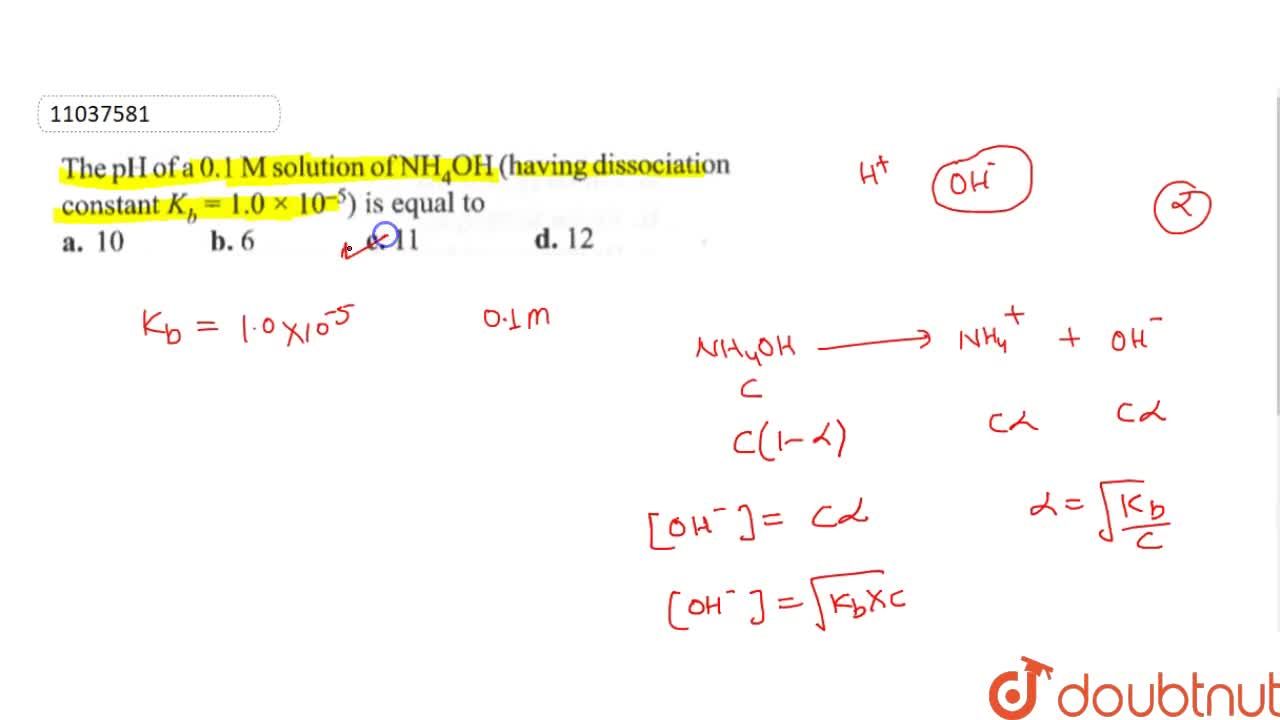
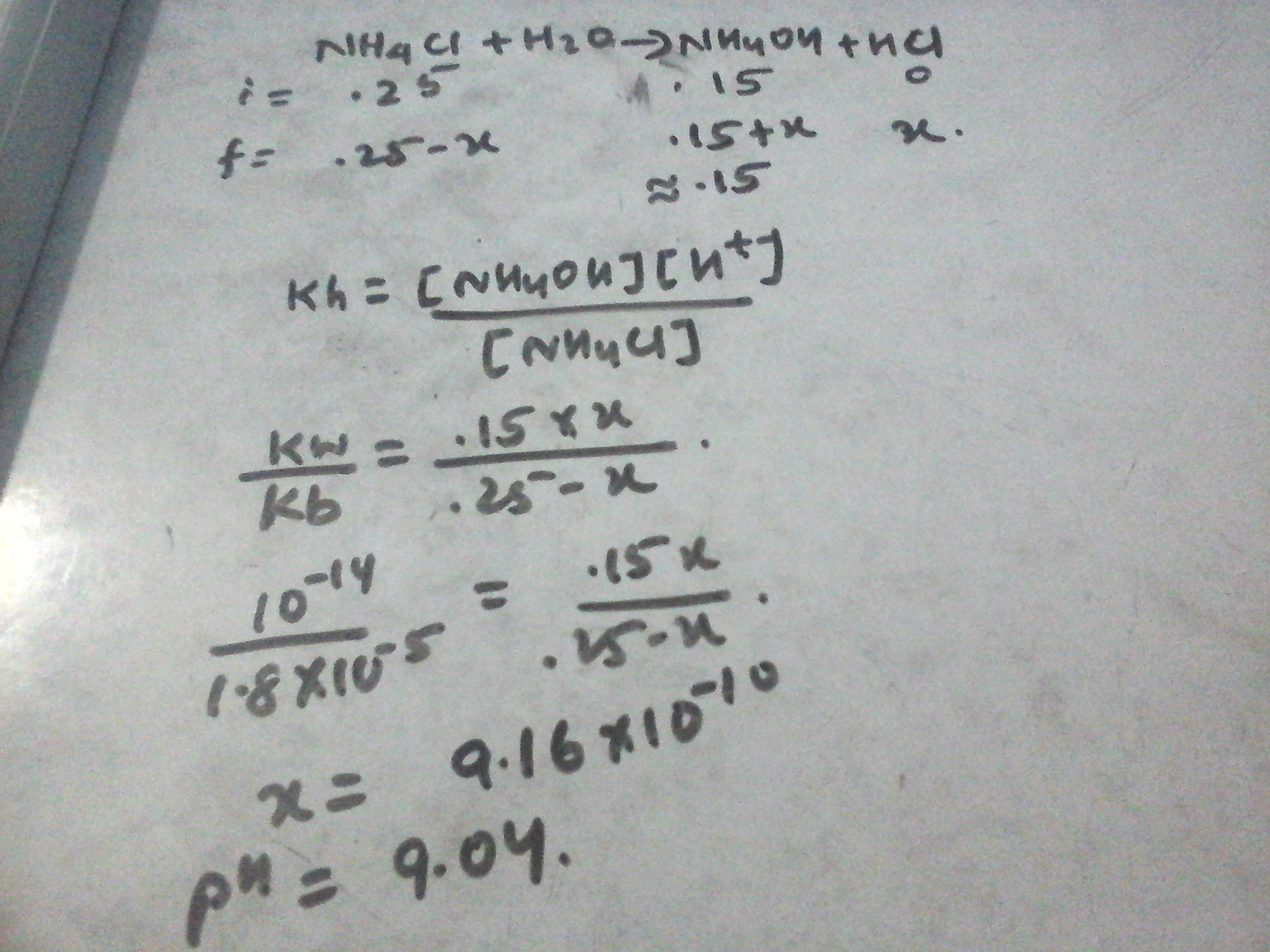A solution of 0.01 M concentration of NH4OH is 2.6% dissociated. - Sarthaks eConnect | Largest Online Education Community

The pH of NH4OH solution is 10.72 in 0.015M solution. Calculate its dissociation constant. (1.83 × 10^-5)
![The pH of a solution containing NH4OH and NH4^+ is 9 . If [NH4^+] = 0.1 M and Ka of NH4^+ is 5 × 10^-10 then what is [NH4OH] ? The pH of a solution containing NH4OH and NH4^+ is 9 . If [NH4^+] = 0.1 M and Ka of NH4^+ is 5 × 10^-10 then what is [NH4OH] ?](https://haygot.s3.amazonaws.com/questions/1835032_827060_ans_d2a61919793549ddbd3f90c517f4ba39.jpg)
The pH of a solution containing NH4OH and NH4^+ is 9 . If [NH4^+] = 0.1 M and Ka of NH4^+ is 5 × 10^-10 then what is [NH4OH] ?
![SOLVED: 2 3 4 5 6 7 8 9 10 11 12 13 14 acidic alkaline neutral Calculation of the pH of 0.IM Ammonium hydroxide (NHAOH): Kb = 1.8*105 [OH:] = VKb € = V18*105*0.1 [OH-] = 1.34*103 pH = -log[H;O"] = log(1.34*10-3) pH =28123 SOLVED: 2 3 4 5 6 7 8 9 10 11 12 13 14 acidic alkaline neutral Calculation of the pH of 0.IM Ammonium hydroxide (NHAOH): Kb = 1.8*105 [OH:] = VKb € = V18*105*0.1 [OH-] = 1.34*103 pH = -log[H;O"] = log(1.34*10-3) pH =28123](https://cdn.numerade.com/ask_images/03307c81d6384600ab2284f2c8cb4a90.jpg)
SOLVED: 2 3 4 5 6 7 8 9 10 11 12 13 14 acidic alkaline neutral Calculation of the pH of 0.IM Ammonium hydroxide (NHAOH): Kb = 1.8*105 [OH:] = VKb € = V18*105*0.1 [OH-] = 1.34*103 pH = -log[H;O"] = log(1.34*10-3) pH =28123

Effects of using NH4OH to regulate pH combined with oxygen-enriched air... | Download Scientific Diagram
71 Calculate the amount of (NH4)2SO4 which must be added to 500 ml of 0.2 (M) NH3 to yield asolution of pH = 9.35. (pKb(NH4OH) = 4.74 )
Calculate the pH of a buffer solution containing 0.45 moles of NH4OH and 0.75 moles of - Sarthaks eConnect | Largest Online Education Community
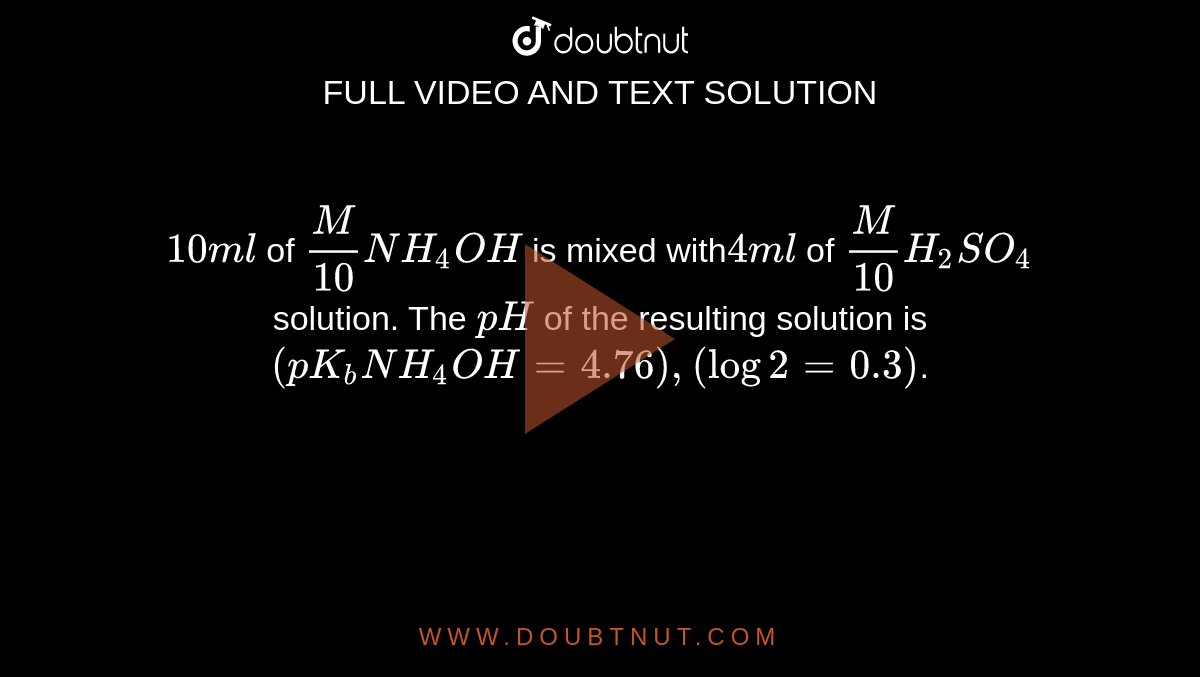
10 ml of M/10 NH(4)OH is mixed with4ml of M/10H(2)SO(4) solution. The pH of the resulting solution is (pK(b)NH(4)OH=4.76), (log2=0.3).

Degree of dissociation of CH3COOH and NH4OH are the same If 0 01M solution of CH3COOH has pH=4; then pH - Chemistry - Equilibrium - 13608053 | Meritnation.com
Welcome to Chem Zipper.com......: What will be the pH of the buffer solution containing 0.15 moles of NH4OH and 0.25 moles of NH4Cl Kb for NH4OH is 1.8x10^-5.
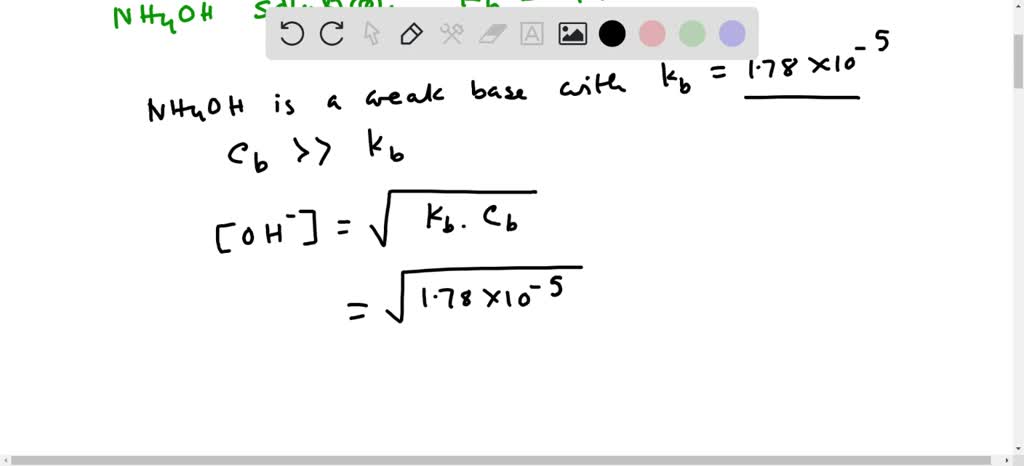
SOLVED: Answer the following equation:Ammonium Hydroxide, NH4OH is a weak base. Calculate the pH of 0.60 M solution of ammonium hydroxide. Kb= 1.78 x 10^-5

The pKb value of ammonium hydroxide is 4.75 An aqueous solution of ammonium hydroxide is titrated with HCl.The pH of the solution at the point where half of the ammonium hydroxide has
![Calculate `[OH^(-)]and %` dissociation of 0.01 M solution of ammonium hydroxide solution. The - YouTube Calculate `[OH^(-)]and %` dissociation of 0.01 M solution of ammonium hydroxide solution. The - YouTube](https://i.ytimg.com/vi/MeVKKmkdPH4/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLALNdqS-oEMY3e24TY1WHJy4IiacA)
Calculate `[OH^(-)]and %` dissociation of 0.01 M solution of ammonium hydroxide solution. The - YouTube