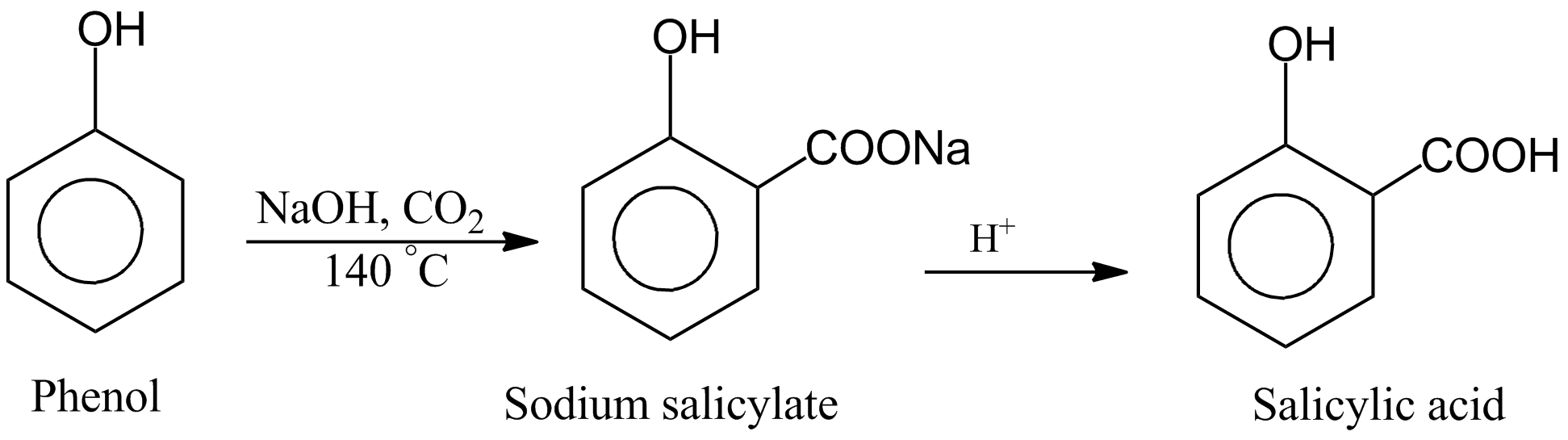
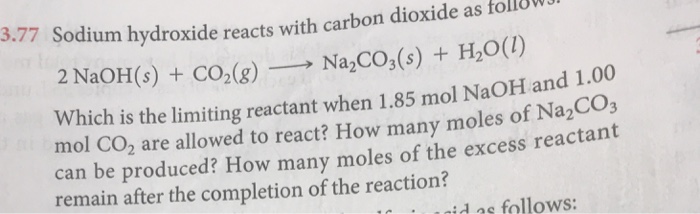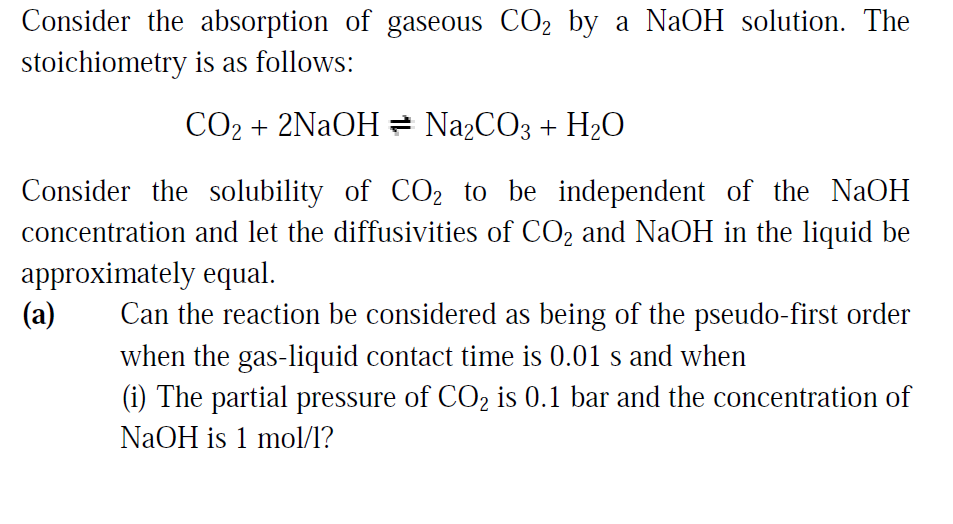How to balance NaOH+CO2=Na2CO3+H2O|Chemical equation NaOH+CO2=Na2CO3+H2O|balance NaOH+CO2=Na2CO3+H2O - YouTube
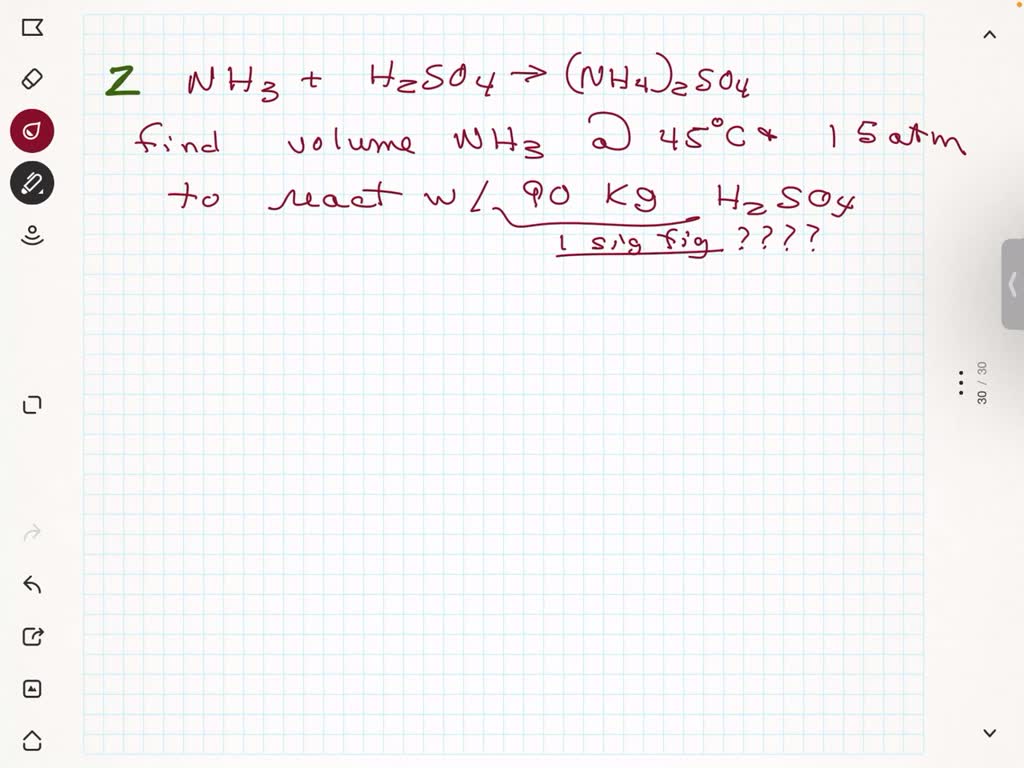
SOLVED: Sodium hydroxide (NaOH) reacts with carbon dioxide (CO2) to form sodium carbonate (Na2CO3) and water. 1.7 moles of NaOH and 1 mole of CO2 are placed in a container. Determine the
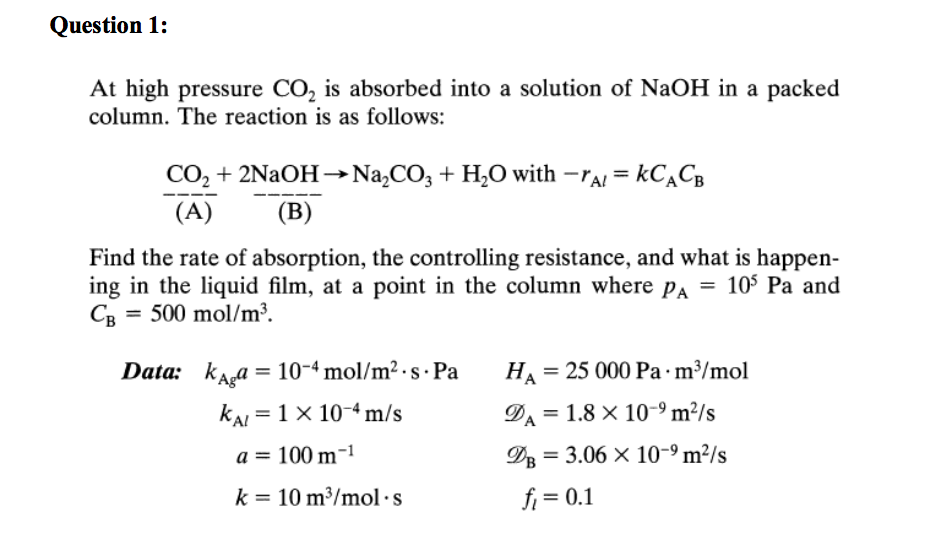
![PDF] Carbon Dioxide Absorption into NaOH Solution in a Cross-flow Rotating Packed Bed | Semantic Scholar PDF] Carbon Dioxide Absorption into NaOH Solution in a Cross-flow Rotating Packed Bed | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d996b4c9767274cb04293287da3efe5f7e1d750f/2-Figure1-1.png)
PDF] Carbon Dioxide Absorption into NaOH Solution in a Cross-flow Rotating Packed Bed | Semantic Scholar

Sustainable and Negative Carbon Footprint Solid-Based NaOH Technology for CO2 Capture | ACS Sustainable Chemistry & Engineering

Schematic of the CO 2 capture system using NaOH aqueous solution: (1) N... | Download Scientific Diagram
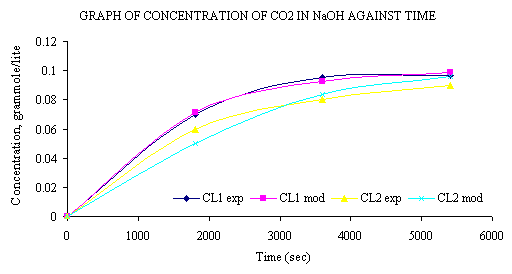
Modeling of a Gas Absorption Column for CO2-NaOH System under Unsteady-State Regime from Leonardo El J Pract Technol