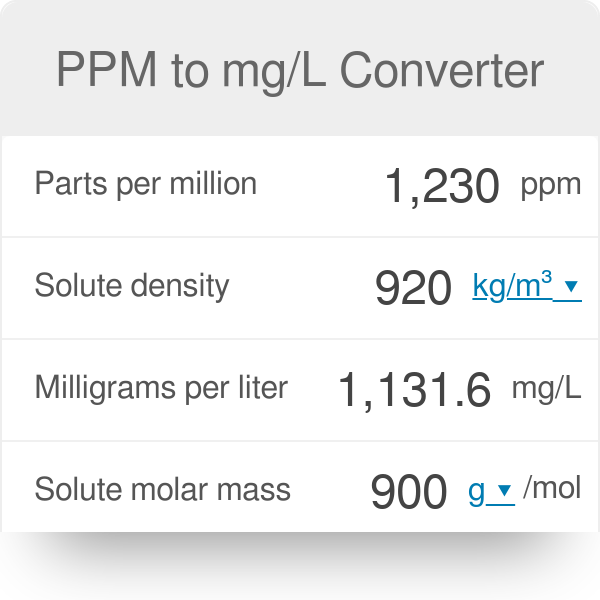
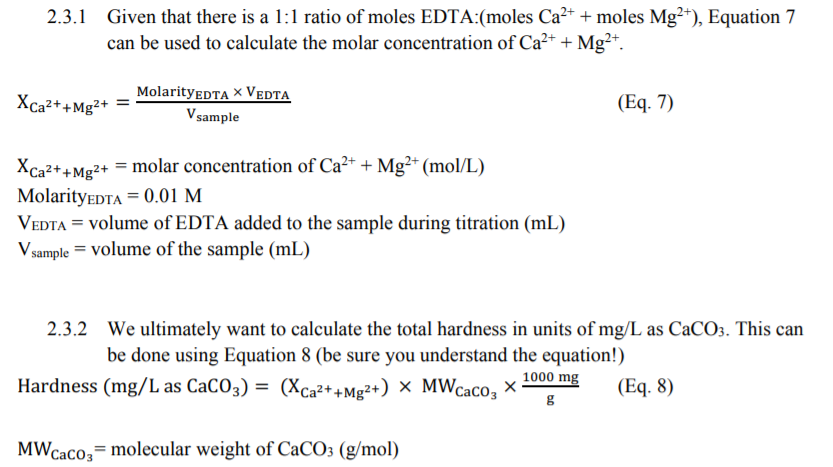
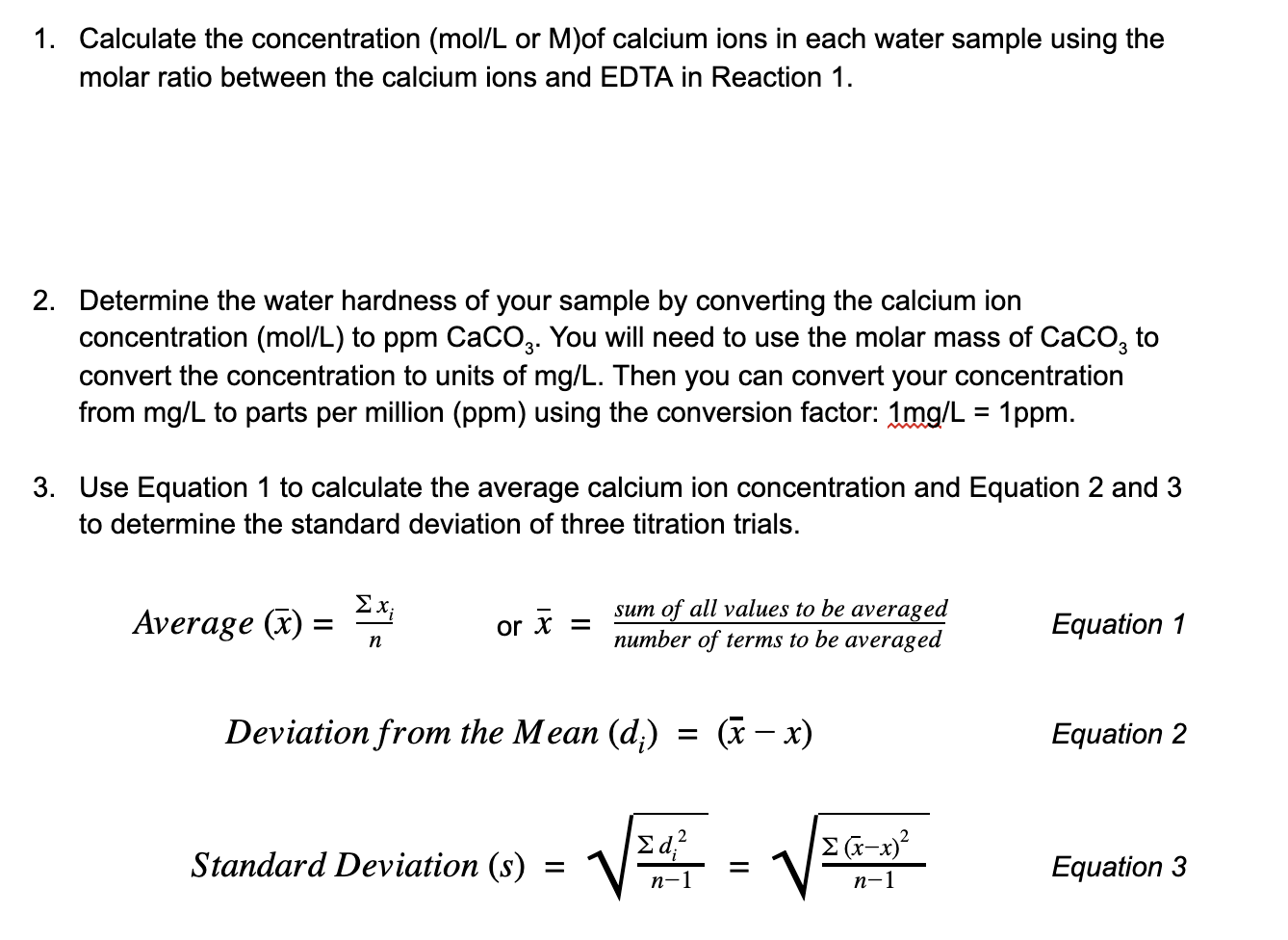
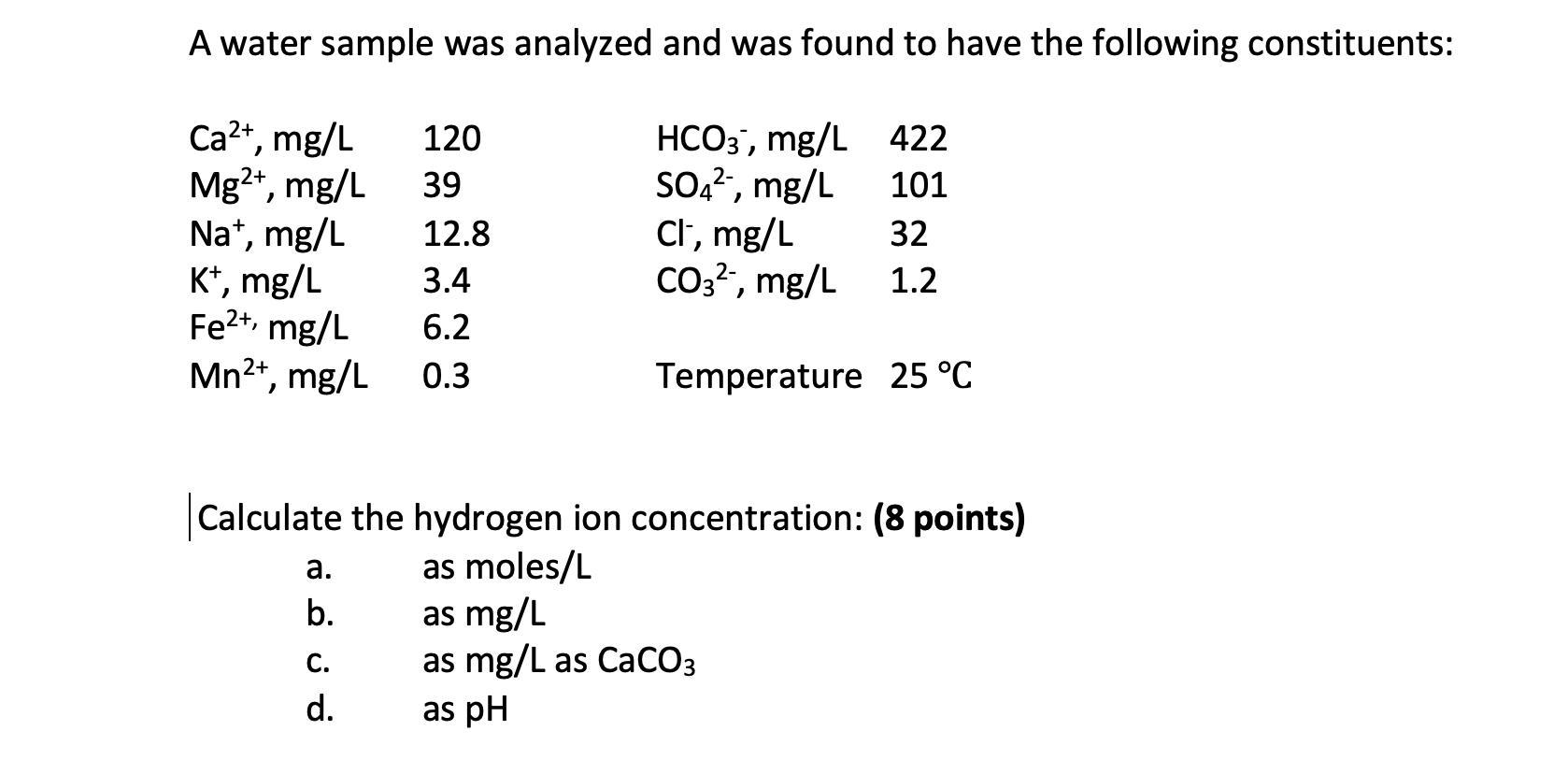
SOLVED: Determine the molar concentration of the dye in the stock dye solution that you used, as follows: (show work for credit) Read the absorbance value AO, at the peak of the
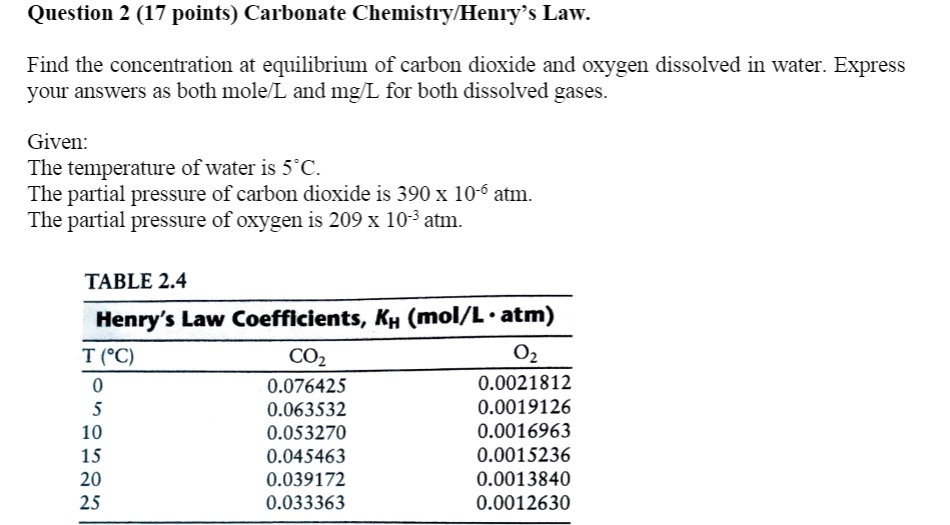
SOLVED: Question 2 (17 points) Carbonate ChemistryMeny Law. Find the concentration at equilibrium of carbon dioxide and oxygen dissolved in water: Express yOur answers as both mole and mg L for both
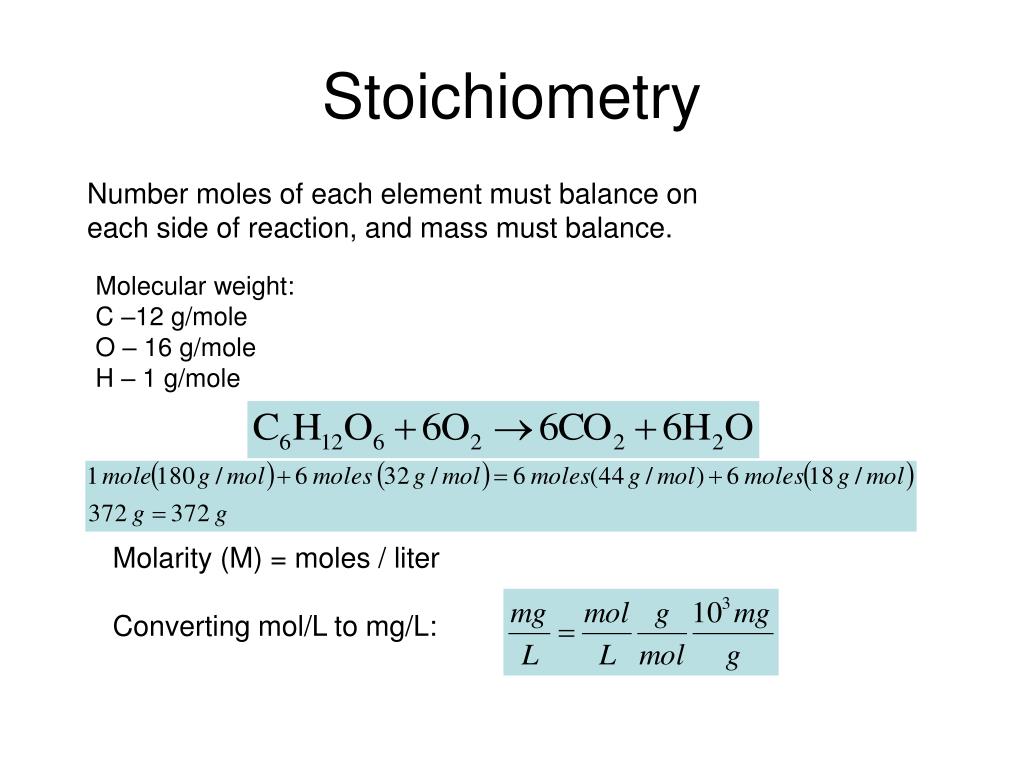
4 A-3 The Mole The mole (abbreviated mol) is the SI unit for the amount of a chemical species. It is always associated with a ch

Question Video: Calculating the Molar Concentration of Mg(OH)₂ Using Data from a Titration Experiment | Nagwa

Speciation graph of Cu(II) (1.0 mg L −1 = 1.57 × 10 −5 mol L −1 ) in... | Download Scientific Diagram