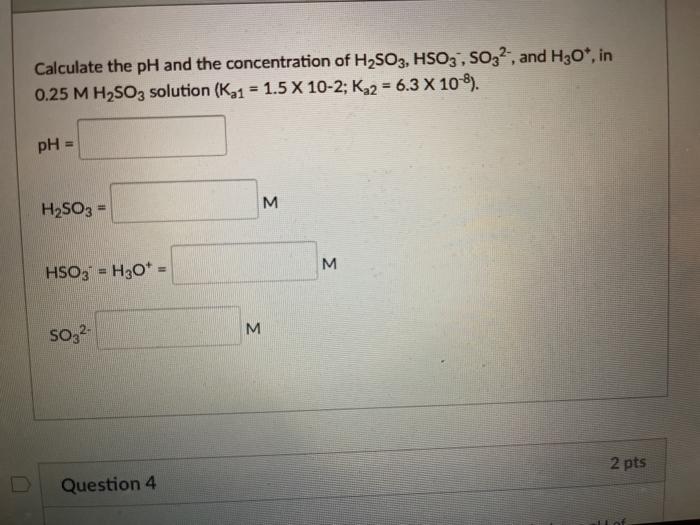
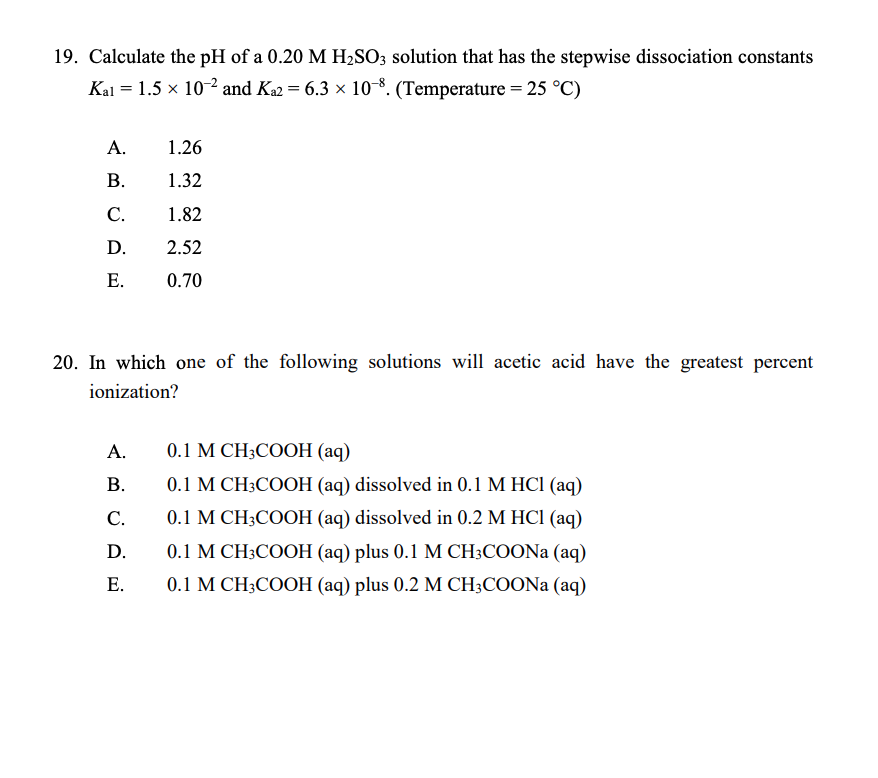
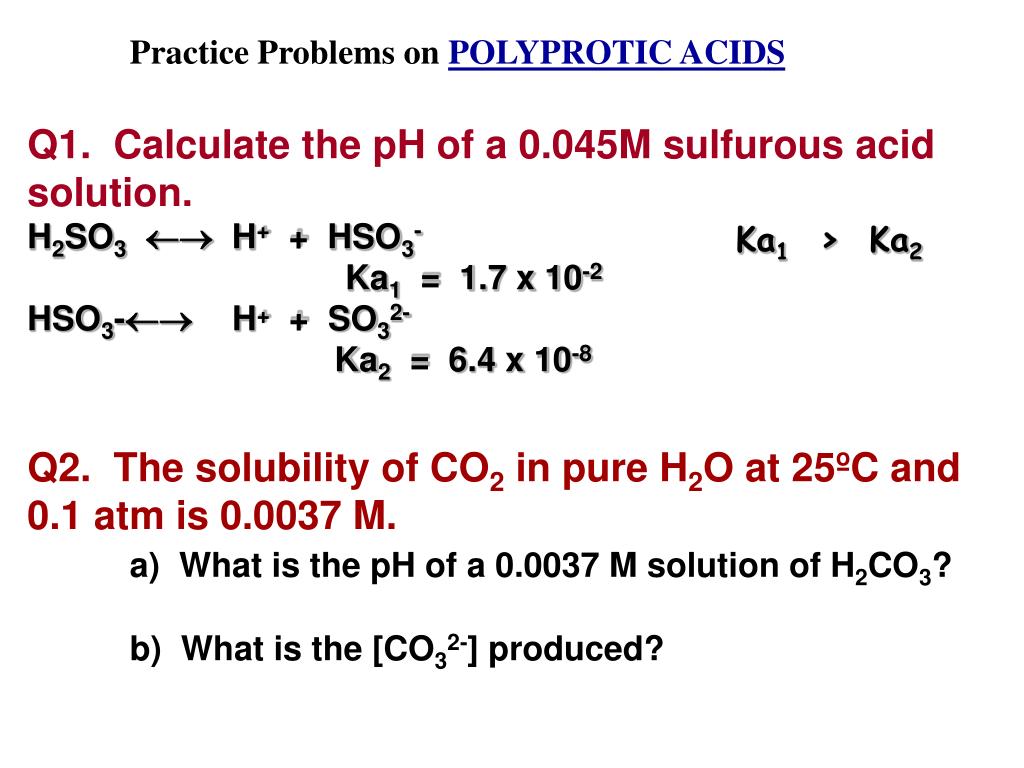
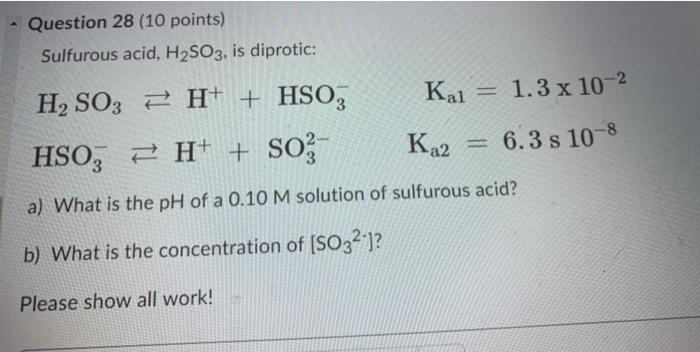
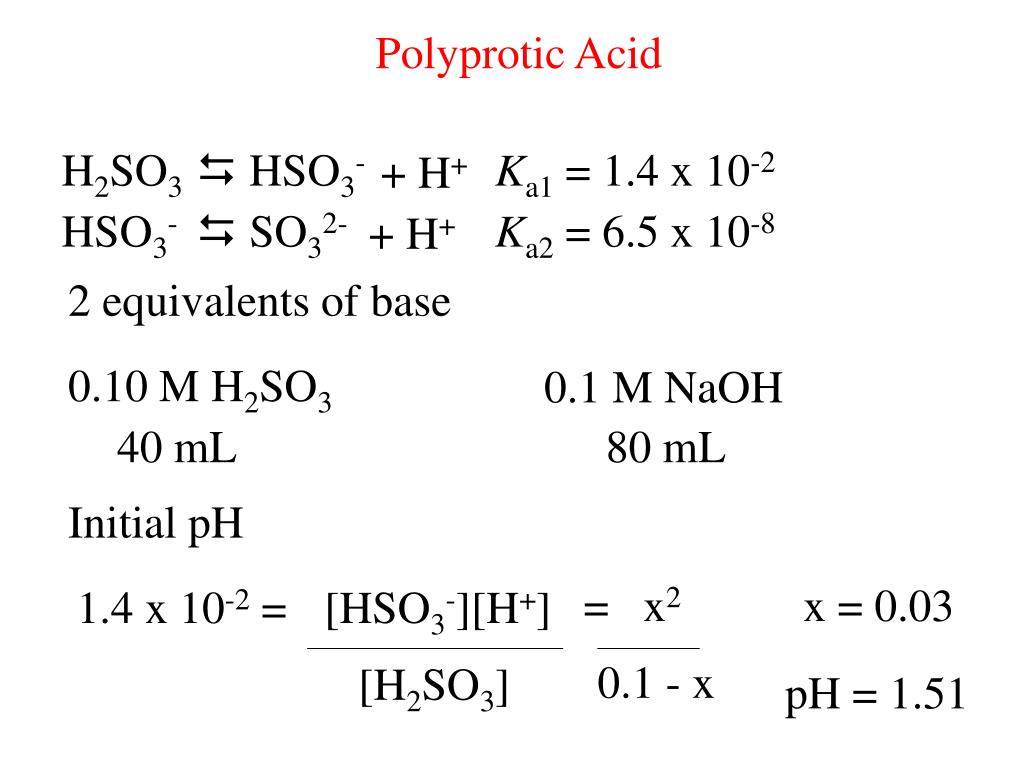
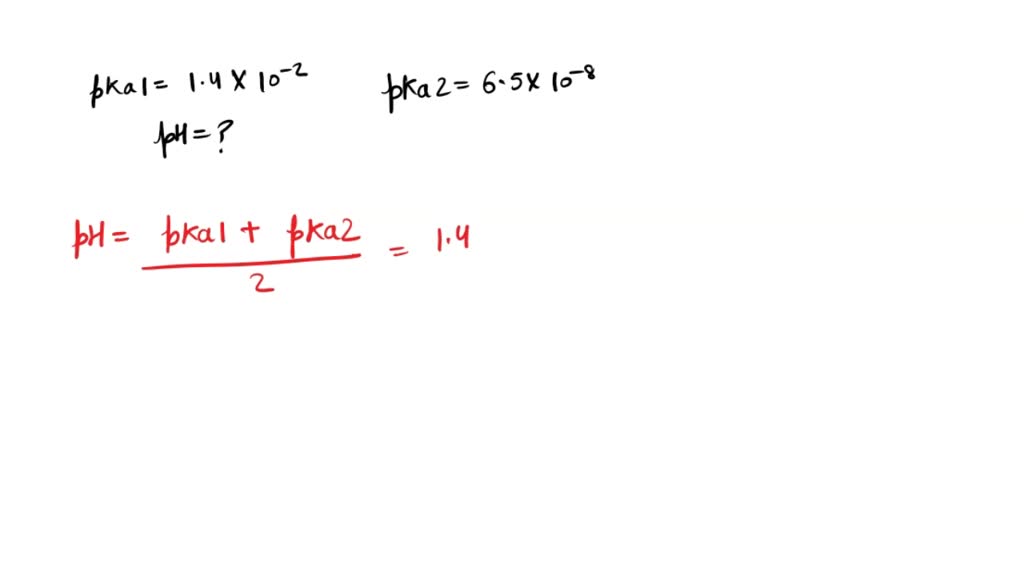Solved) Sulfurous acid, H2SO3 has acid dissociation constants Ka1 = 1.5 × 10-2 and Ka2 = 6.3 × 10-8. What is
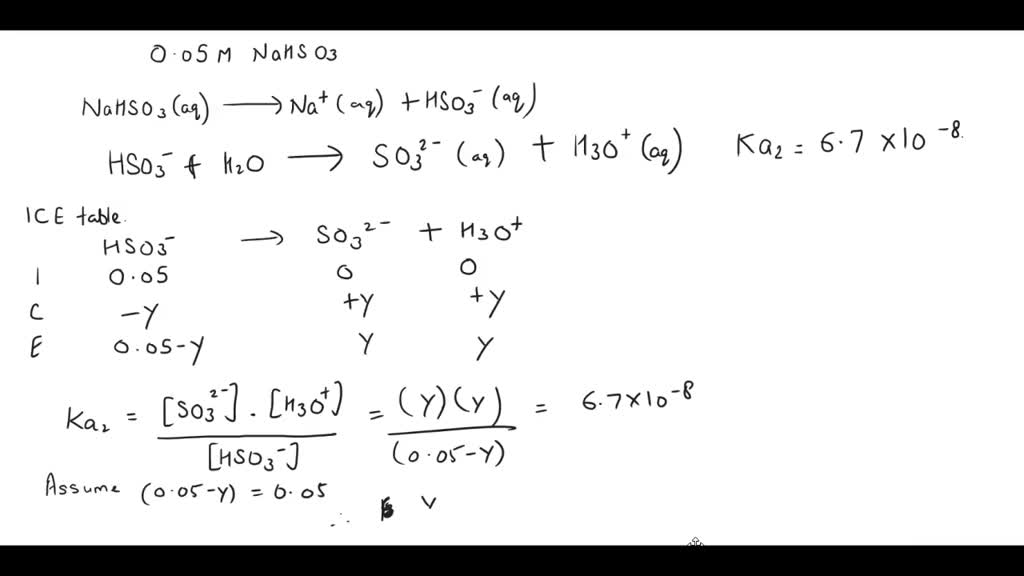
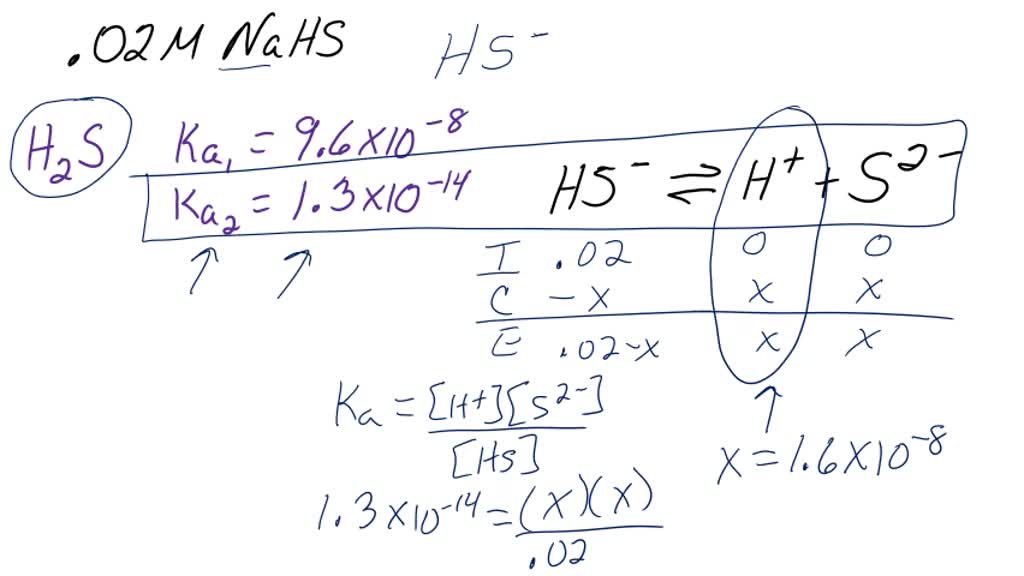
SOLVED: Find the pH and the concentrations of H2SO3, HSO3 - and SO3 2- in 0.05 M NaHSO3 solution. Ka1 and Ka2 for H2SO3 is 1.39 X 10-2 and 6.71 X 10-8 respectively.
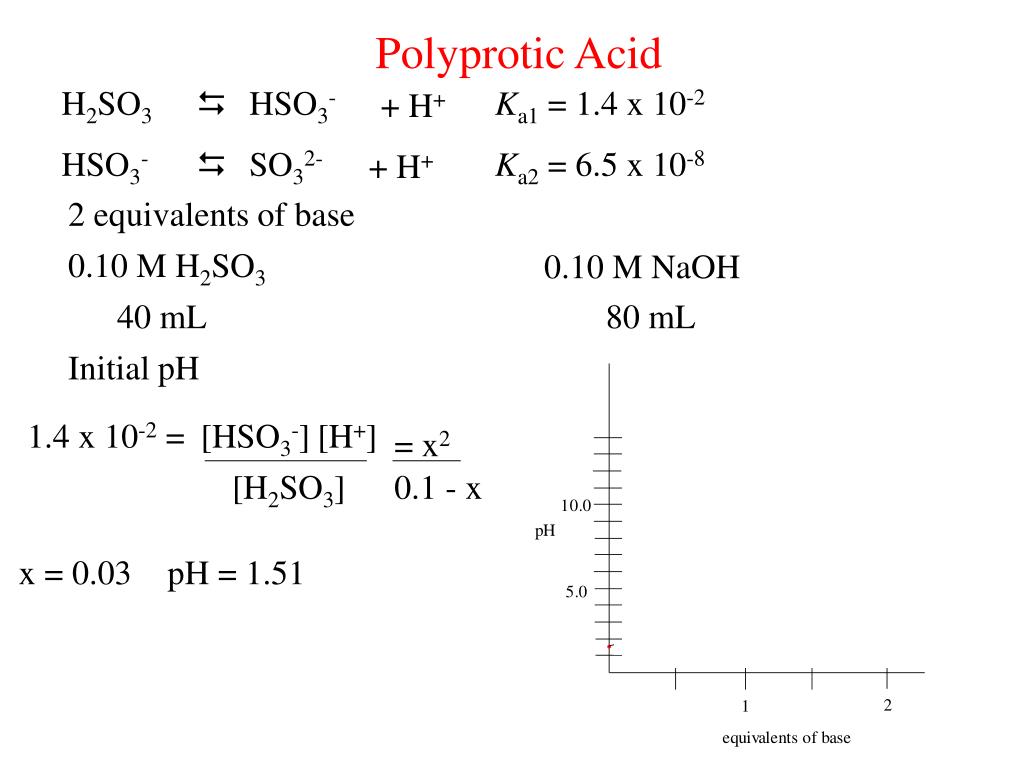
Sulphurous acid (H2SO3) has Ka1 = 1.7 × 10^–2 and Ka2 = 6.4 × 10^–8. The pH of 0.588 M H2SO3 is ..... - Sarthaks eConnect | Largest Online Education Community
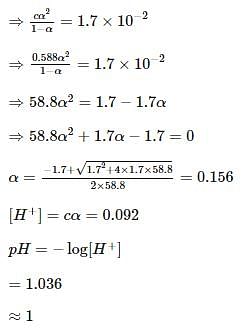
Sulphurous acid (H2SO3) has Ka1 = 1.7 × 10-2 and Ka2 = 6.4 × 10-8. The pH of 0.588 M H2SO3 is ______. (Round off to the nearest integer)Correct answer is '1'.
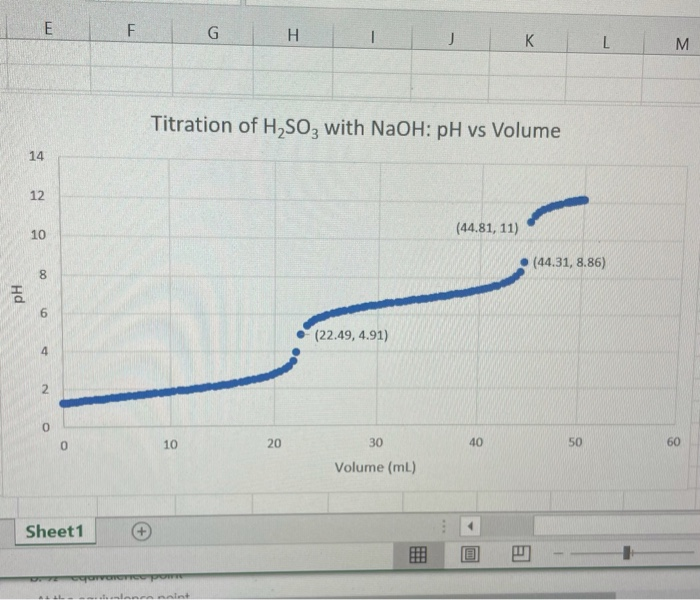
The fraction of S(IV) species (αHSO − 3 , αSO 2− 3 , and αH 2 SO 3 ) as... | Download Scientific Diagram

Sulphurous acid (H(2)SO) has Ka(1) = 1.7 xx 10^(-2) and Ka(2)=6.4 xx 10^(-8). The pH of 0.588 M H(2)SO(3) is (Round off to the Nearest Integer).

OneClass: 1) Which of the following is not a conju gate acid-base pair? A) H2SO3/HSO3 E) None of the ...

Volcanic sulfur dioxide (SO2) becomes sulfurous acid (H2SO3) in water... | Download Scientific Diagram
![SOLVED: QUESTION 2 sulfurous acid (H2SO3) is diprotic weak acid. Calculate the equilibrium value of 0.2M [H2SO3 ] Use Ka values from Appendix C (Hint: quadratic equation may be needed to find SOLVED: QUESTION 2 sulfurous acid (H2SO3) is diprotic weak acid. Calculate the equilibrium value of 0.2M [H2SO3 ] Use Ka values from Appendix C (Hint: quadratic equation may be needed to find](https://cdn.numerade.com/ask_images/5b620c57a4a34e3093345181929fd912.jpg)
SOLVED: QUESTION 2 sulfurous acid (H2SO3) is diprotic weak acid. Calculate the equilibrium value of 0.2M [H2SO3 ] Use Ka values from Appendix C (Hint: quadratic equation may be needed to find