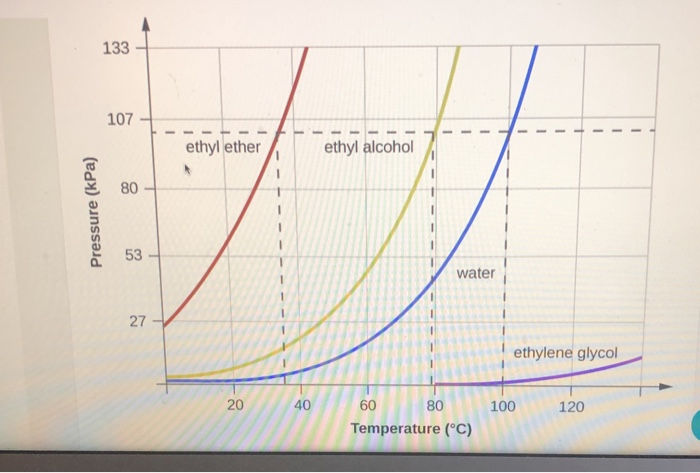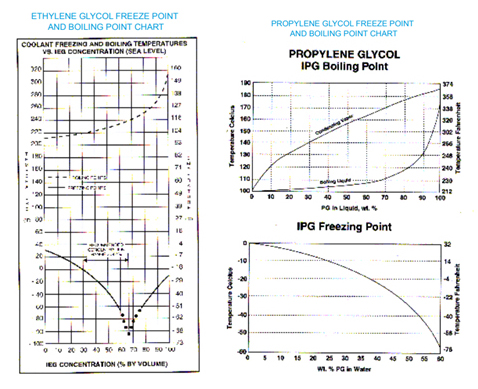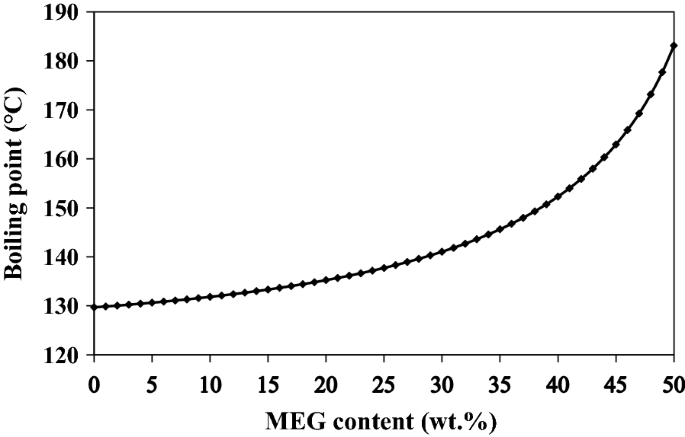
Ethylene glycol elimination in amine loop for more efficient gas conditioning | BMC Chemistry | Full Text
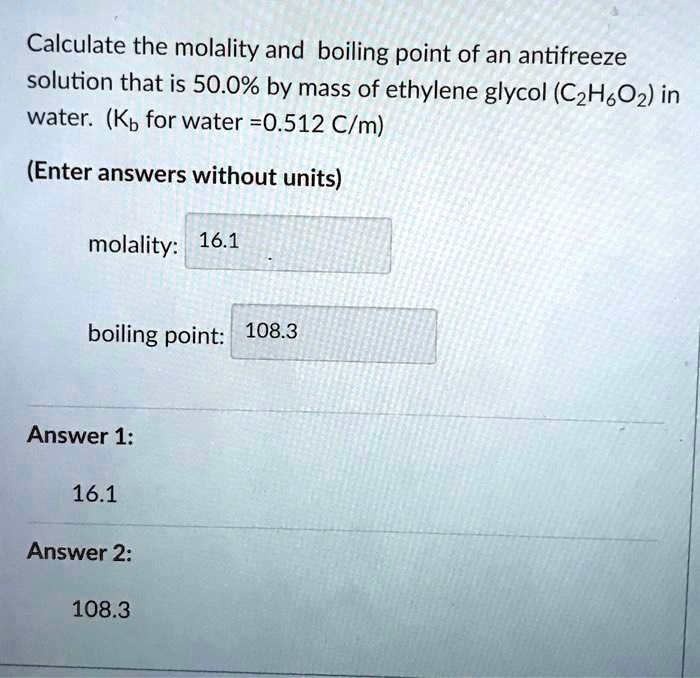
SOLVED: Calculate the molality and boiling point of an antifreeze solution that is 50.0% by mass of ethylene glycol (CzHsOz) in water. (Kb for water =0.512 C/m) (Enter answers without units) molality:

Thermal and catalytic decomposition of aqueous ethylene glycol mixtures by film boiling - ScienceDirect