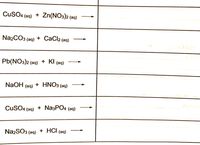
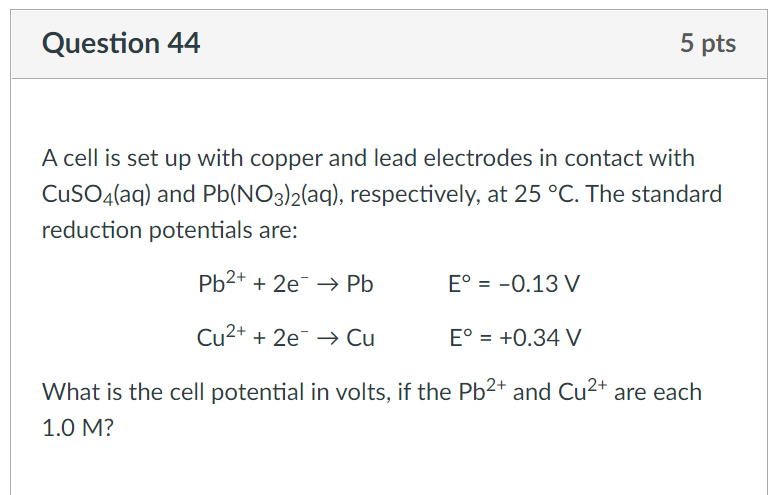
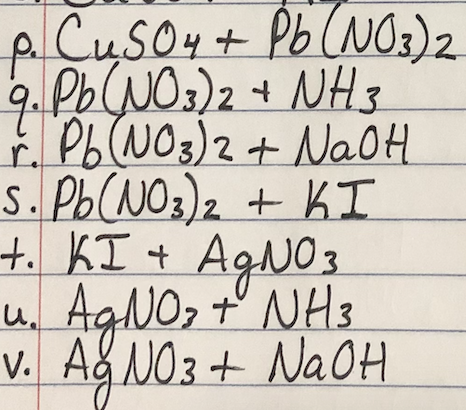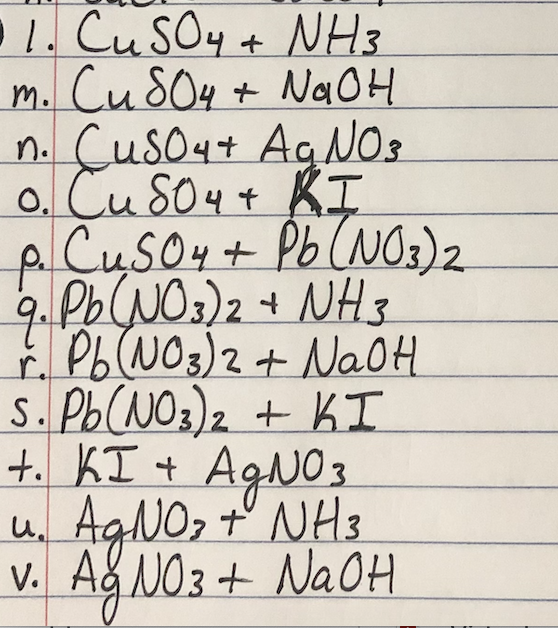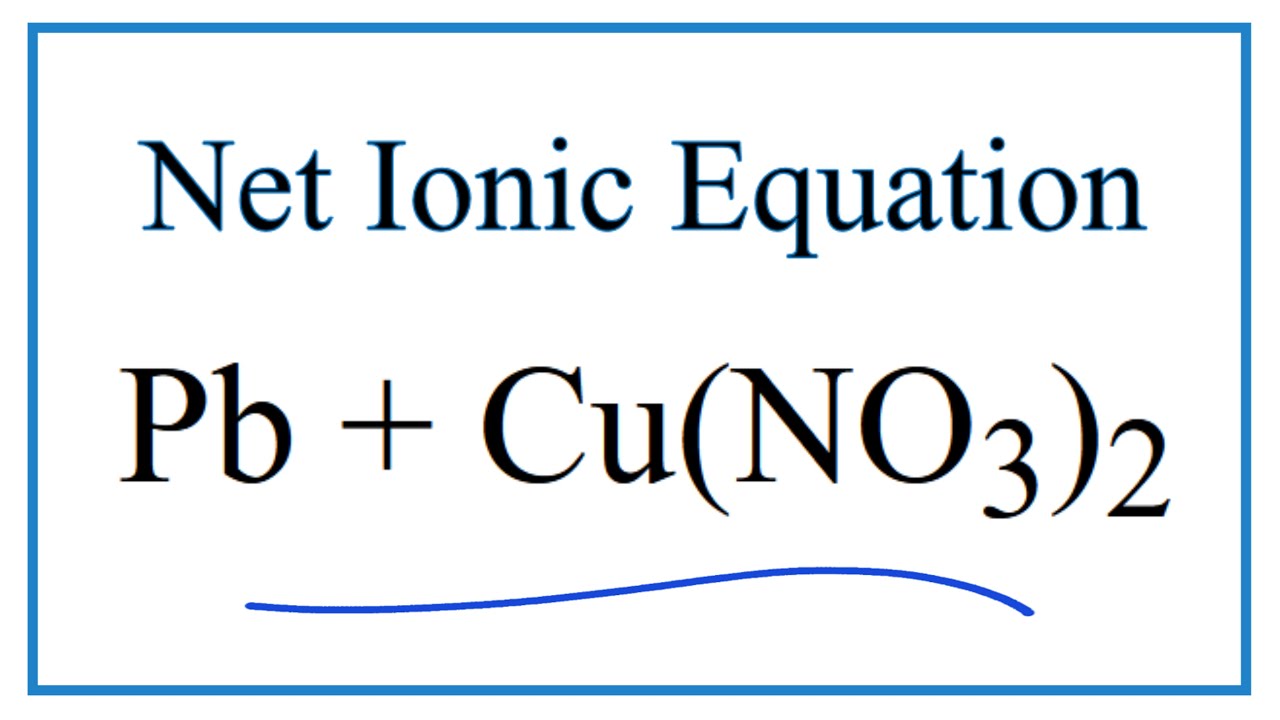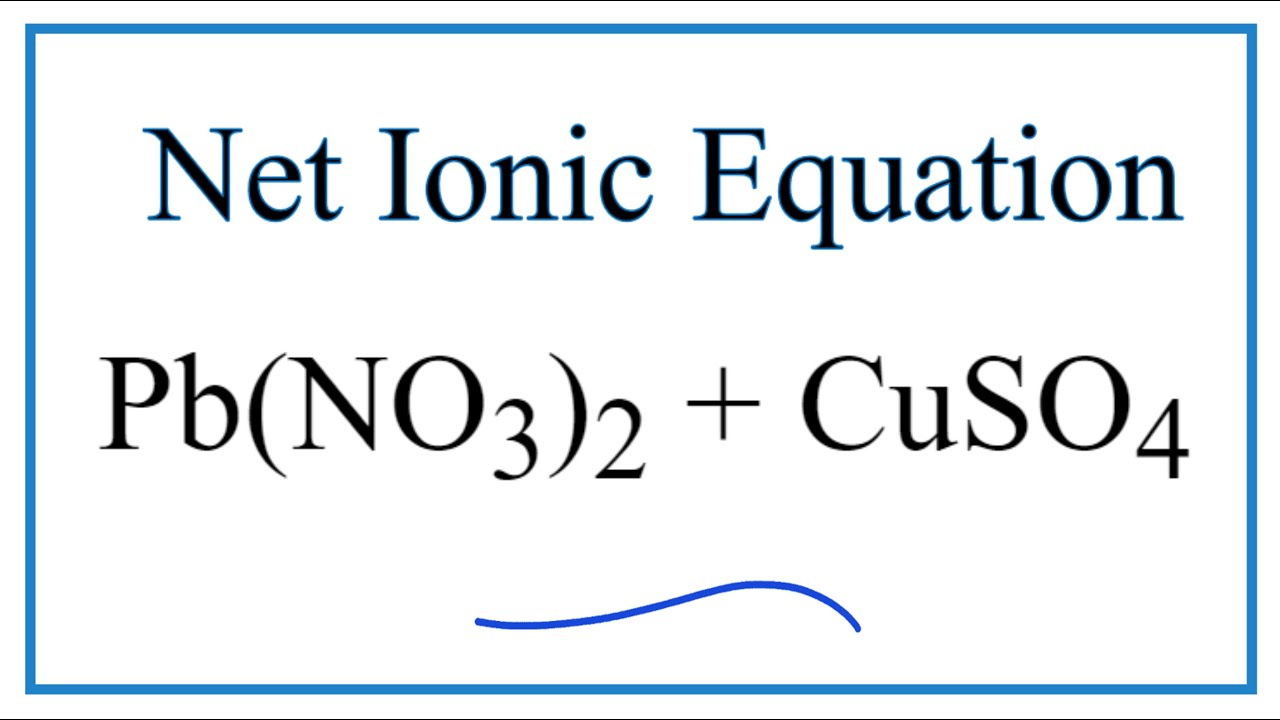Consider a spontaneous electrochemical cell between Cu and Al . Predict what would happen if excess concentrated NaOH were added to the cell with copper ions and a precipitate forms.Standard Potential (V)Reduction

Pb(NO3)2 +CuSO4 =PbSO4 + Cu(NO3)2 Balanced Equation||Led nitrate plus Copper sulphate Balanced Equa. - YouTube

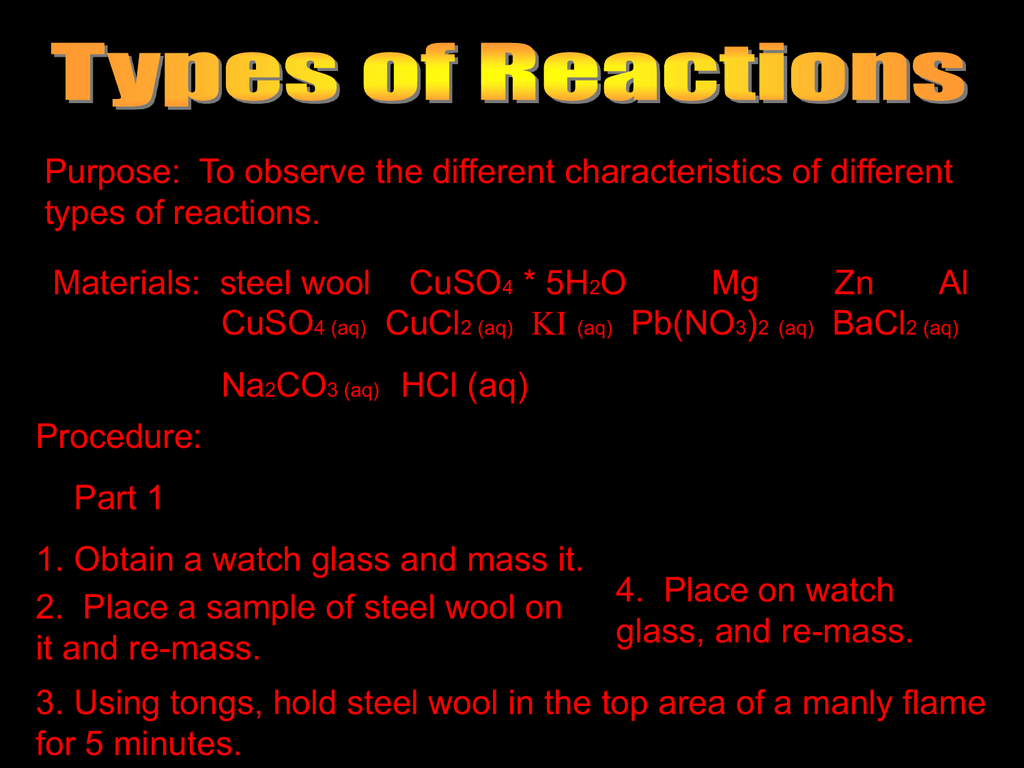
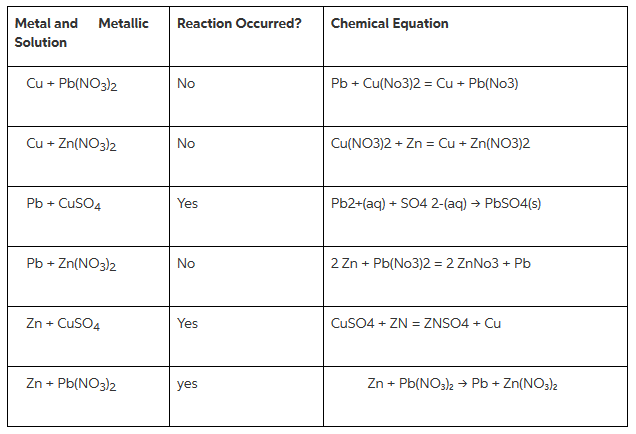
Chapter 10 Jeopardy!!!! Can use Periodic Tables, activity series and solubility rules only!!!! (no book, no notes, etc.) Write answer LARGE & LEGIBLE on. - ppt download

How to Balance Pb(NO3)2 + CuSO4 = PbSO4 + Cu(NO3)2 | Lead (II) nitrate plus Copper (II) Sulfate - YouTube

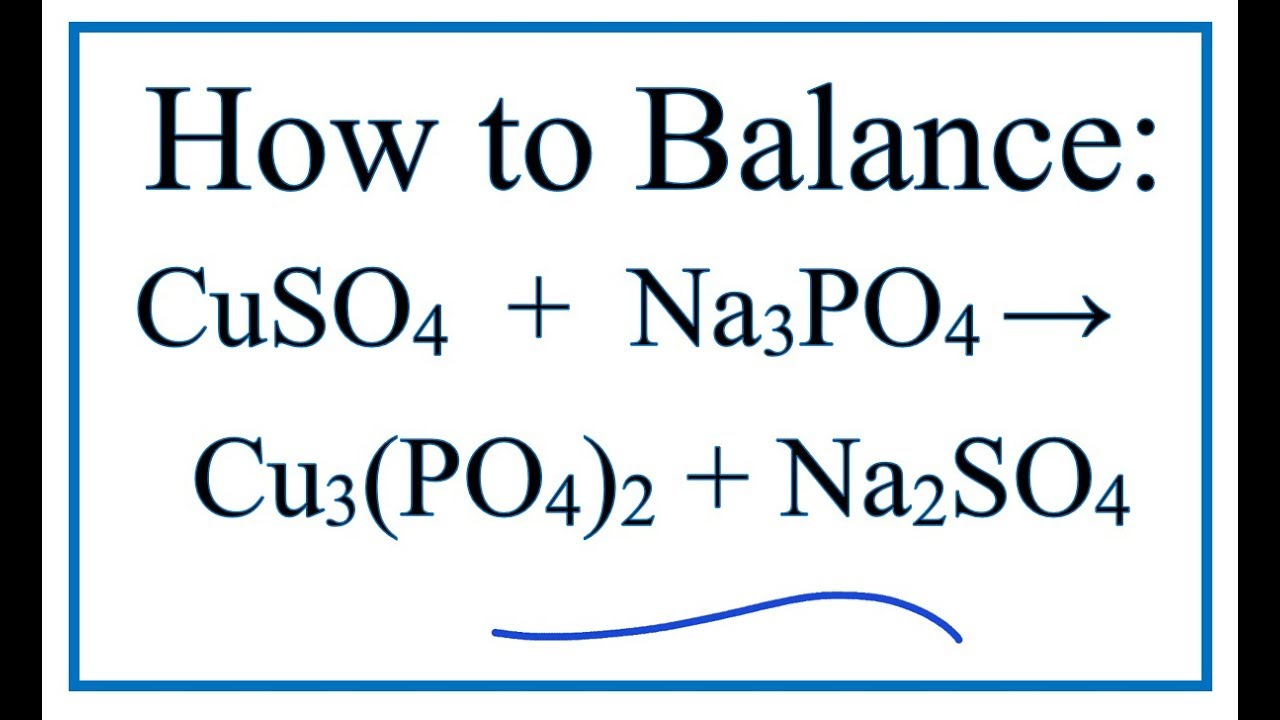
Podaj równanie reakcji w postaci jonowej oraz skróconej: CuSo4 + Na3Po4 Cucl2 + Pb(No3)2 CuSo4 + Pb - Brainly.pl