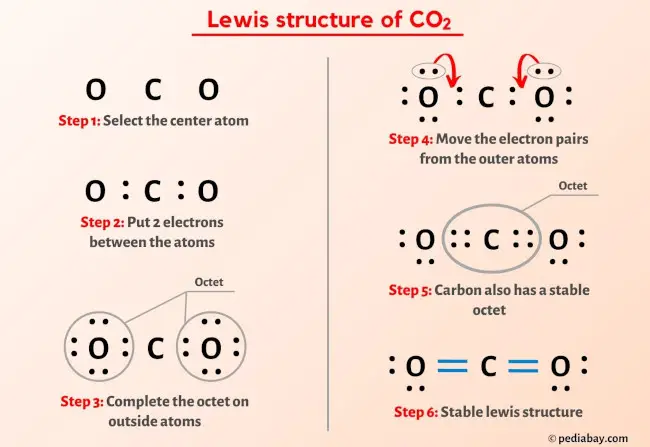
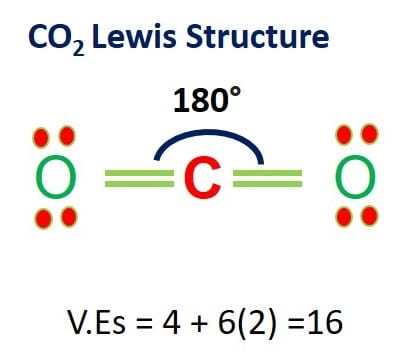
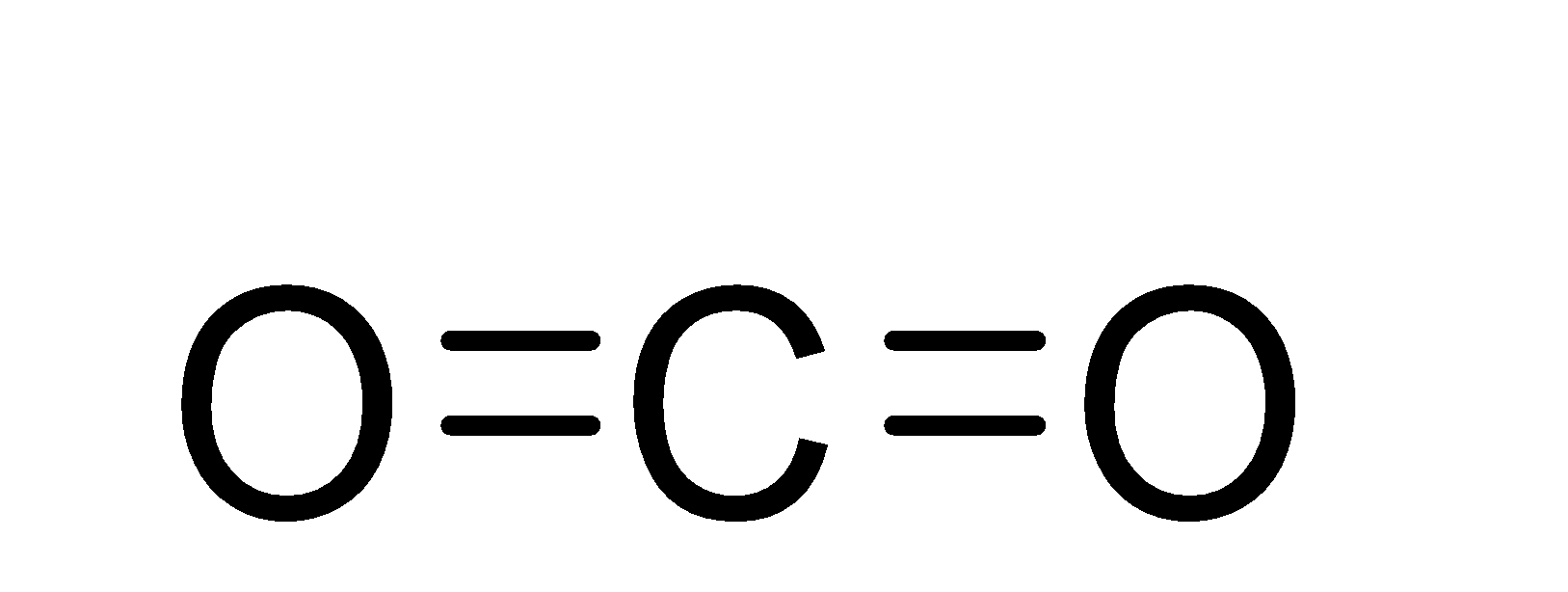![CO2 = CO + [O]: recent advances in carbonylation of C–H bonds with CO2 - Chemical Communications (RSC Publishing) CO2 = CO + [O]: recent advances in carbonylation of C–H bonds with CO2 - Chemical Communications (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/D0CC00547A)
CO2 = CO + [O]: recent advances in carbonylation of C–H bonds with CO2 - Chemical Communications (RSC Publishing)
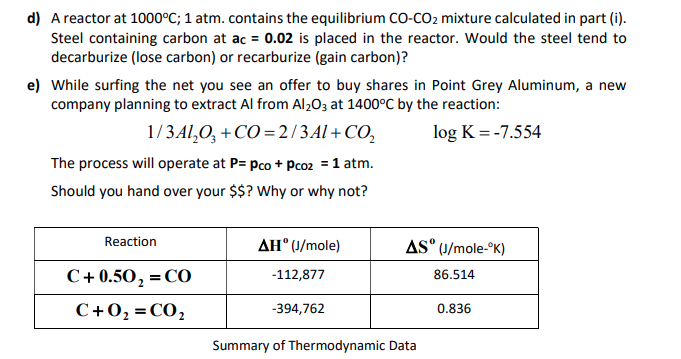
22g of CO2 at 27 celsius is mixed in a closed container with 16 gram of O2 at 37 Celsius if both gases are considered as Ideal kinetic theory of gases then

A sample of carbon dioxide has a volume of 26.5 mL at 20 degree C and 624 torr. How many grams of CO2 are in the sample? | Socratic
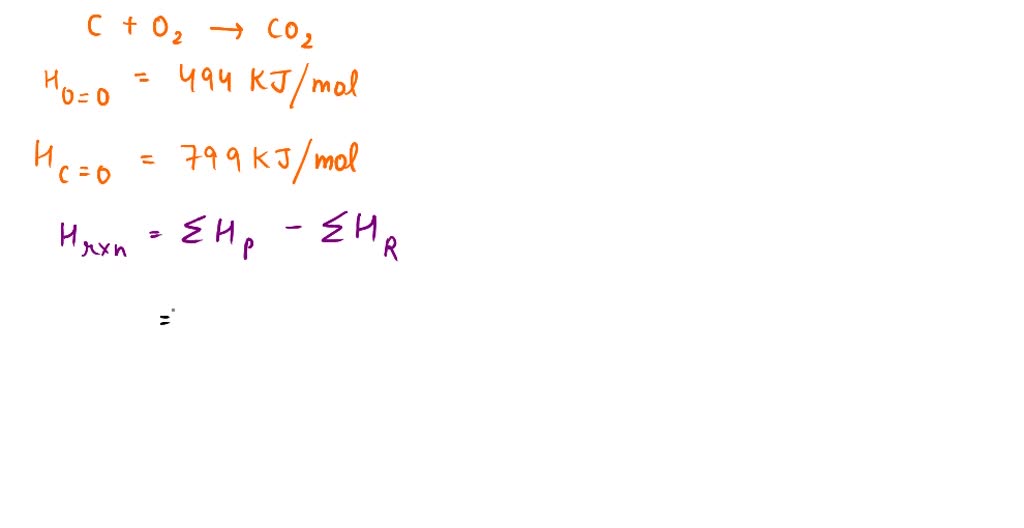
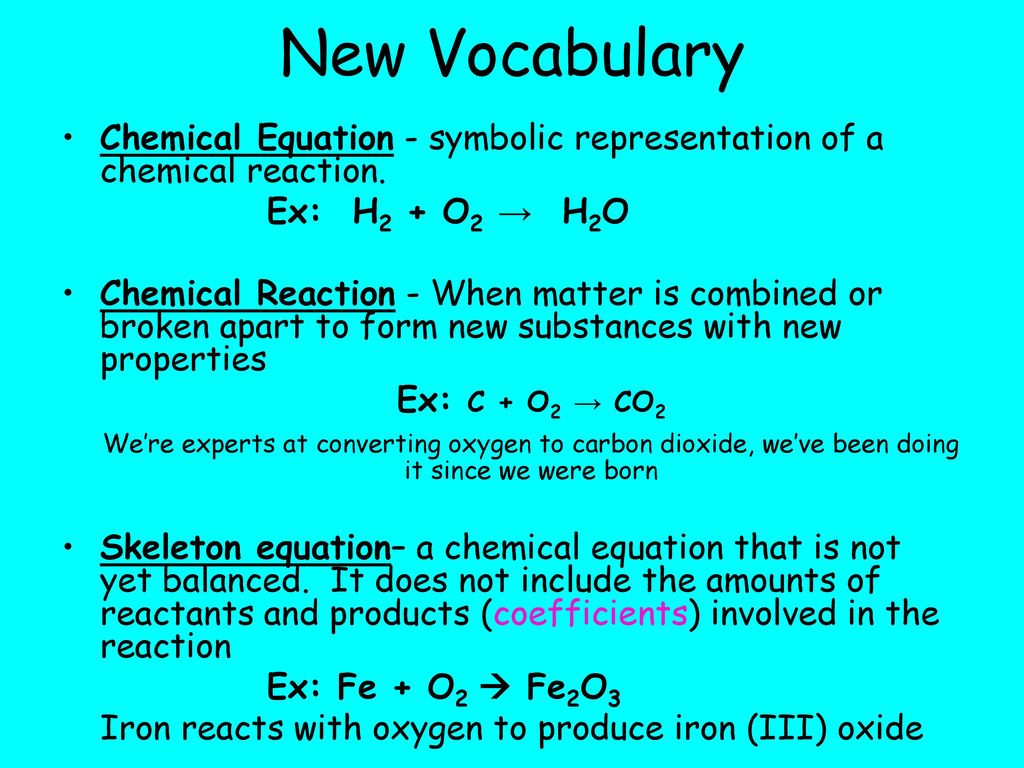
SOLVED: Given: C + O2 → CO2 Bond Bond Energy (kJ/mol) C=O 799 O=O 494 Calculate the enthalpy change for the chemical reaction. The change in enthalpy for the given reaction is