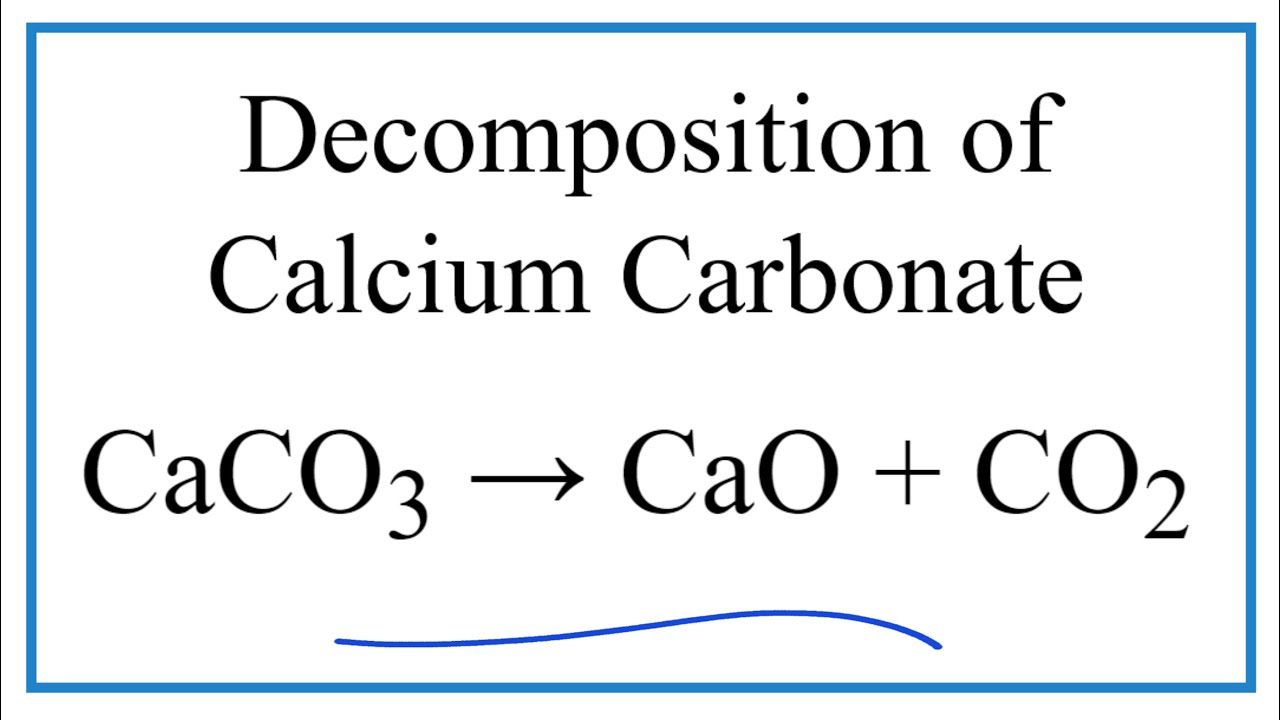
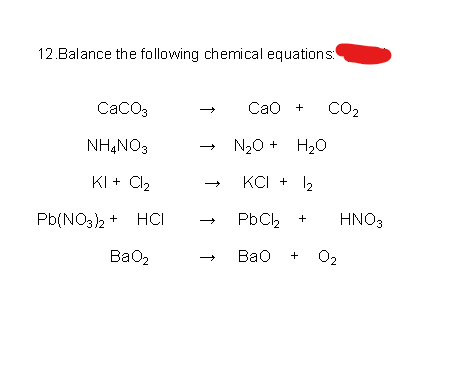
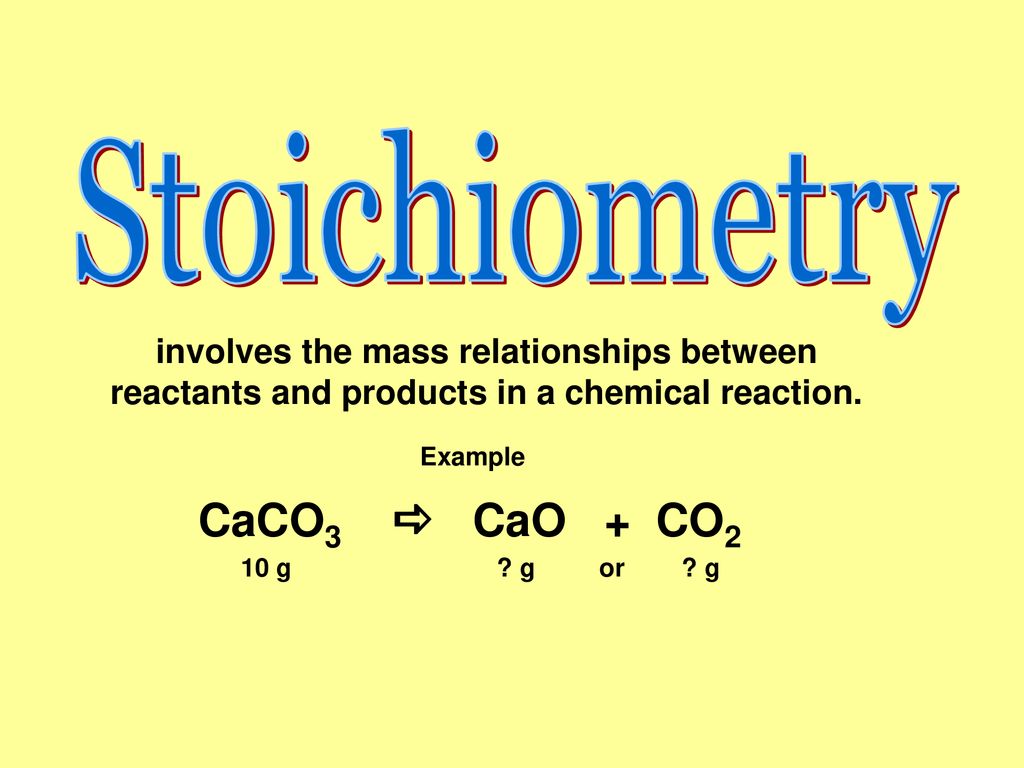
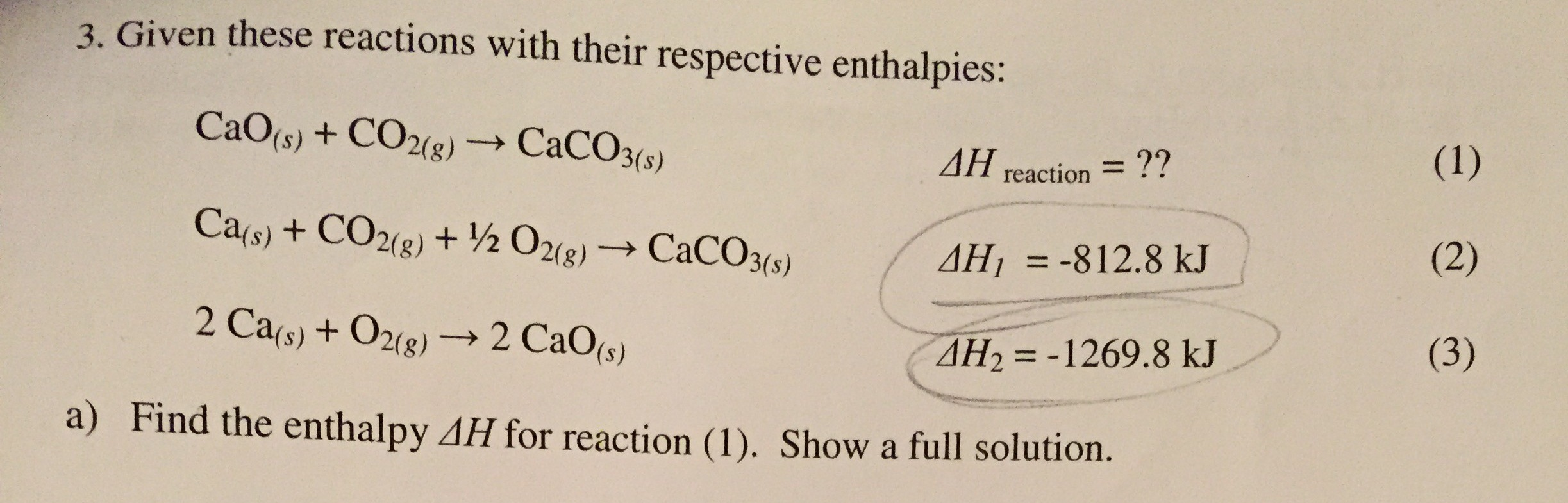Effect of steam on CaO regeneration, carbonation and hydration reactions for CO2 capture - ScienceDirect

CaCO3 = CaO + CO2From the above equation calculate the amount of carbon dioxide in grams liberated by - Brainly.in

For the reaction: CaCO3(s) ⇌ CaO(s) + CO2(g). Kp= 1 atm at 927°C. If 20g of CaCO3 were kept in a10 liter vessel at 927°C, then calculate percentage of CaCO3 remaining at
If 220 grams of calcium oxide (CaO) reacts with 50L of carbon dioxide (CO2), what mass of calcium carbonate (CaCO3) is produced? - Quora
8.CaCO3(s)=CaO(s) +CO2(g).If Kp for the above reaction at 1200K is 1.5 atm.Some CaCO3 is taken in a 10 litre closed vessel,then the number of moles of CaO at equilibrium is (Take R=1/12
![SOLVED: Question 2 (5 points) Saved What is the expression for the equilibrium constant? CaO(s) + CO2(g) CaCO: (8) [CaOJICO2] [CaCO:] b) Keq [CaCO:] [CaOJcoz] c) Keq ` [CO2] [CO2 Keq Keq ` SOLVED: Question 2 (5 points) Saved What is the expression for the equilibrium constant? CaO(s) + CO2(g) CaCO: (8) [CaOJICO2] [CaCO:] b) Keq [CaCO:] [CaOJcoz] c) Keq ` [CO2] [CO2 Keq Keq `](https://cdn.numerade.com/ask_images/928a15231b094c709901e0e2efd39d00.jpg)