What is the pH of a 0.402 M aqueous solution of NaCH3COO? Ka (CH3COOH) = 1.8x10-5 | Homework.Study.com
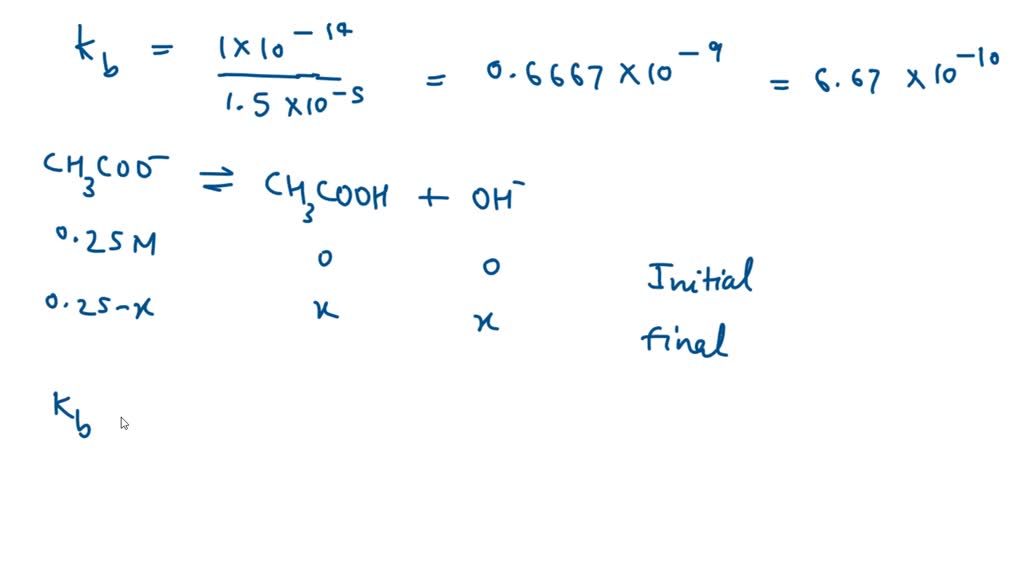
SOLVED: What is the pH of a 0.25 M of a solution of CH3COONa salt solution (for Acetic Acid Ka = 1.5 x 10-5).

for 50ml of 0.05M ch3cooh how much vol of 0.1M ch3coona must be added to get buffer sol.. - Brainly.in
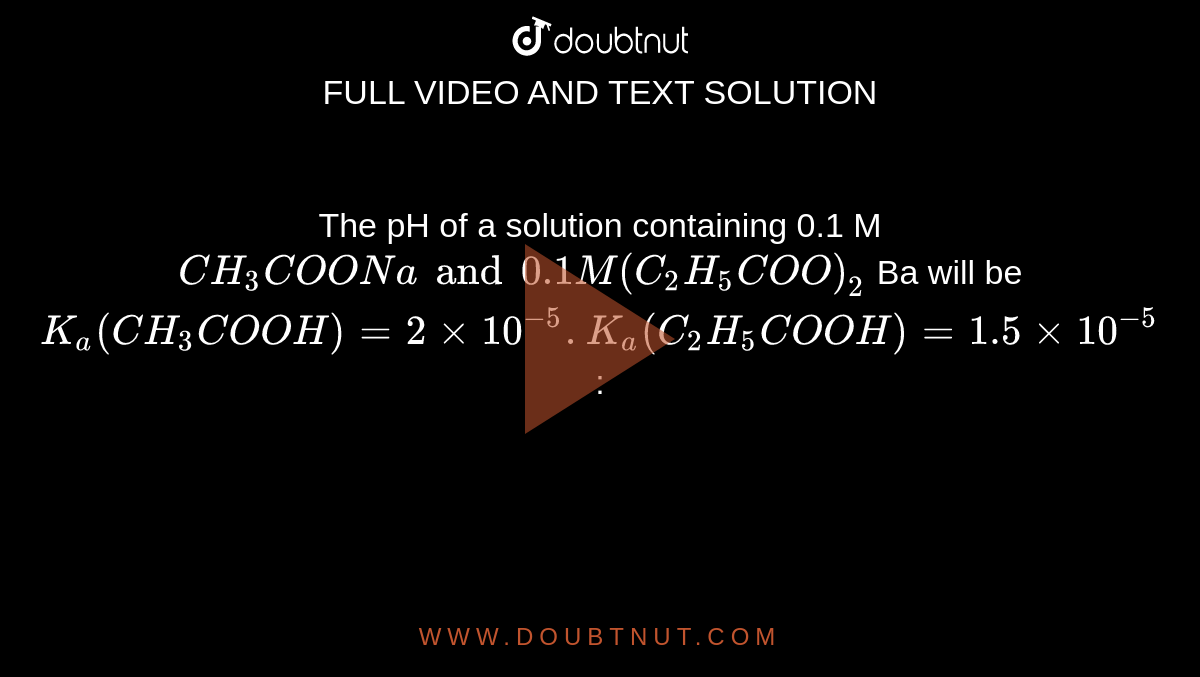
The pH of a solution containing 0.1 M CH3COONa and 0.1 M (C2H5COO)2 Ba will be Ka(CH3COOH)=2xx10^(-5). Ka(C2H5COOH)=1.5xx10^(-5):
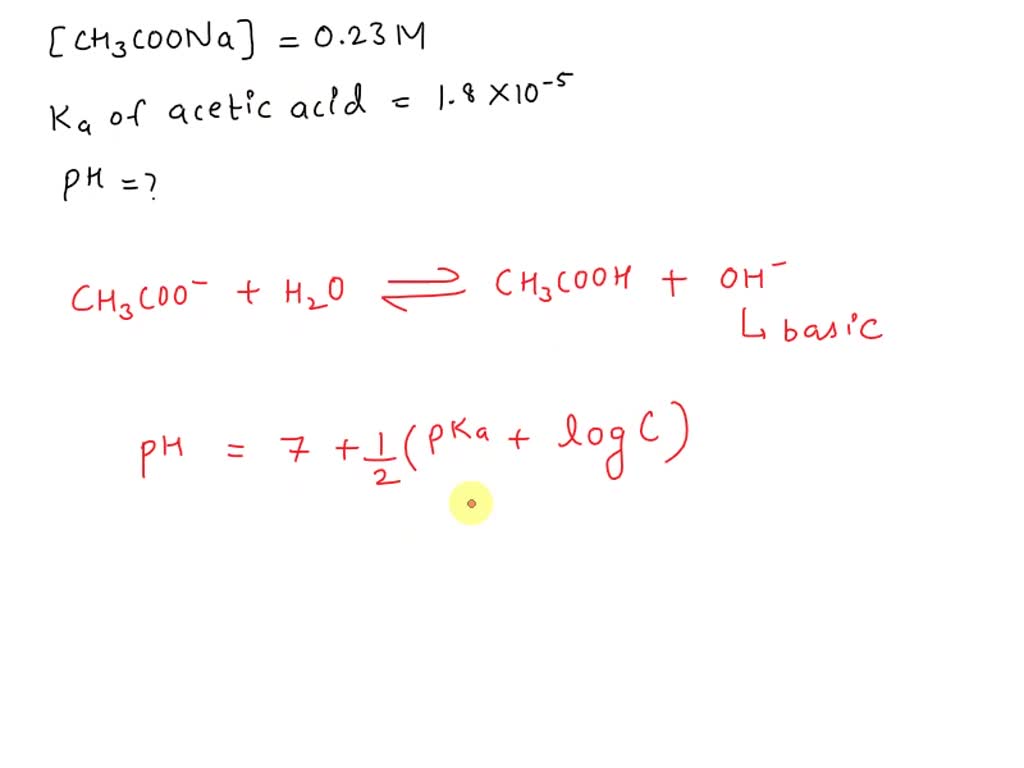
SOLVED: Determine the pH of a 0.23 M solution of sodium acetate (CH3COONa) at 25°C. (Ka of acetic acid = 1.8 × 10−5.) pH = ?
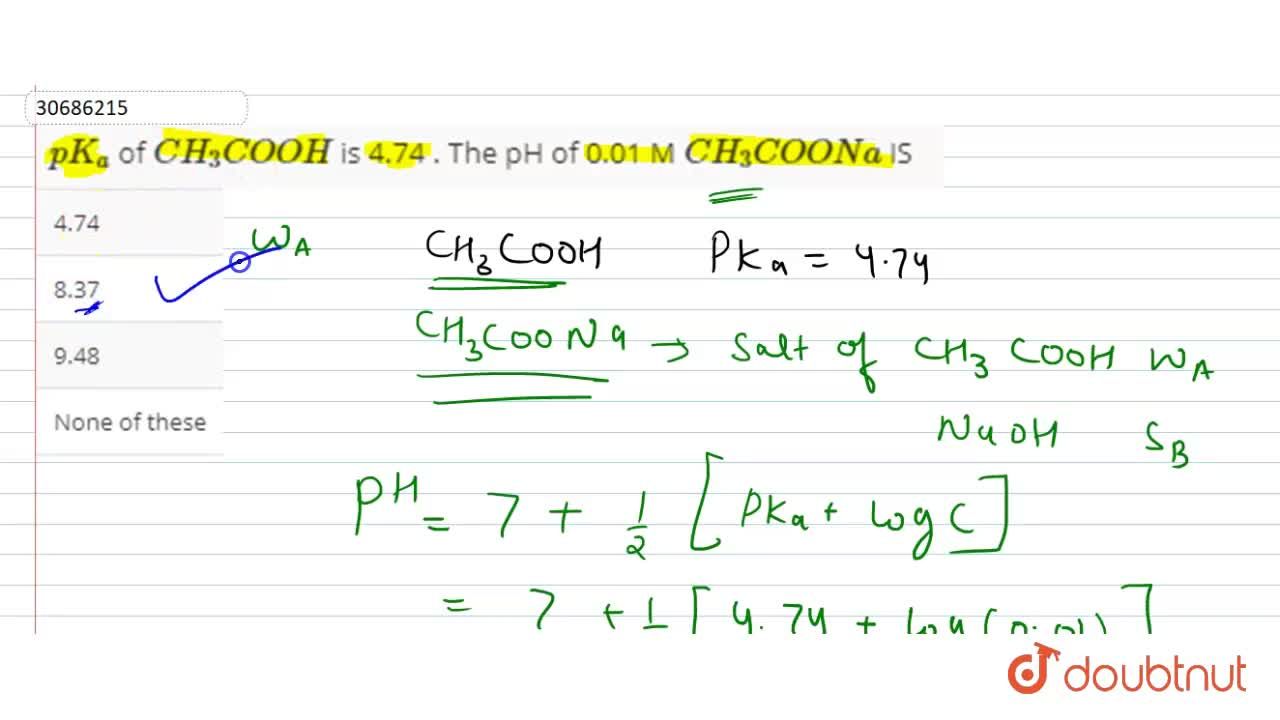
pH of a buffer containing 6.0 g of CH3COOH and 8.2 g of CH3COONa in 1 L of water is (pKa = 4.74) : - Sarthaks eConnect | Largest Online Education Community
Sodium acetate, CH3COONa, is used for developing photographs. Find the value of Kb for the acetate ion. Then calculate the pH of a solution that contains 12.5 g of sodium acetate dissolved
A buffer solution of pH = 4.7 is prepared from CH3COONa and CH3COOH. Dissociation constant of acetic acid is - Sarthaks eConnect | Largest Online Education Community
For preparing a buffer of pH 6 by mixing sodium acetate and acetic acid the ratio of the concentration of salt and acid should be ( Ka=10^ 5)
8. Which of the following increasing order of pH of 0.1M solutions of the compound A HCOONH4 B CH3COONH4 C CH3COONa D NH4Cl is correct

Calculate the ratio of a pH of a solution containing 1 mole of CH3COONa 1 mole of HCl per litre and of other solution containing 1 mole CH3COONa 1 mole of acetic
Welcome to Chem Zipper.com......: What is the pH of 0.10 M CH3COONa solution. Hydrolysis constant of sodium acetate is 5.6 × 10-10 ?
Electrolysis of 50L aqueous solution of CH3COONa was done by passing 2F of electricity. The pH of the solution and the products obtained at anode and cathode are respectively
![The pH of a buffer solution prepared by mixing 50 ml of 0.2M CH3COOH and 25 ml of CH3COONa is 4.8 . What is the concentration of CH3COONa ? [pKa of CH3COOH = 4.8] The pH of a buffer solution prepared by mixing 50 ml of 0.2M CH3COOH and 25 ml of CH3COONa is 4.8 . What is the concentration of CH3COONa ? [pKa of CH3COOH = 4.8]](https://dwes9vv9u0550.cloudfront.net/images/4471247/3dcf789c-3f9c-4640-89f9-bb92dfd5e087.jpg)
The pH of a buffer solution prepared by mixing 50 ml of 0.2M CH3COOH and 25 ml of CH3COONa is 4.8 . What is the concentration of CH3COONa ? [pKa of CH3COOH = 4.8]

Calculate the pH of a 0.39 M CH3COONa solution. (Ka for acetic acid = 1.8 × 10−5.) | Wyzant Ask An Expert