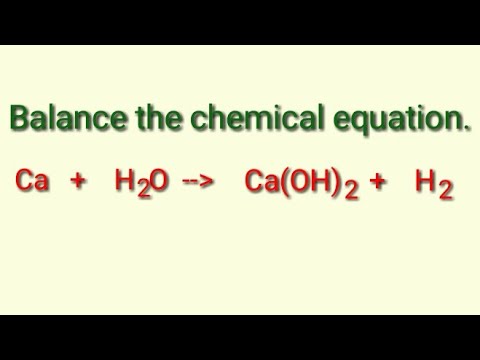
From the following data, the heat of formation of Ca(OH)2(s) at 18^∘C is .... Kcal .(i) CaO(s) + H2O (l) = Ca(OH)2(s); H18^∘C = - 15.26 Kcal ..... ( ii) H2O(l) = H2(g) +

Cuántos gramos de Ca(OH)2 se formaran por la reacción de 28 gramos de óxido de calcio (CaO)?. CaO + H2O → - Brainly.lat

SOLVED: For the reaction CaO (s) + H2O (l) → Ca(OH)2 (s) ∆H0 = -64.8 kJ. How many grams of CaO must be reacted by this reaction to release 1050 kJ of

How to balance CaO+H2O=Ca(OH)2|Chemical equation CaO+H2O=Ca(OH)2|Reaction balance CaO+H2O=Ca(OH)2 - YouTube

Schematic diagram of CaO/Ca(OH)2 chemical heat pump: (a) heat storing... | Download Scientific Diagram
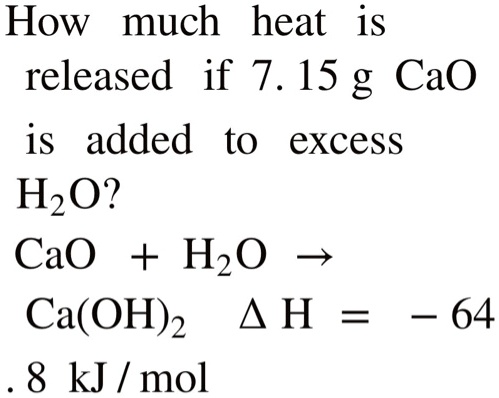
SOLVED: How much heat is released if 7. 15 g CaO is added to excess H2O2 CaO + H2O Ca(OH)2 4 H = 64 8 kJ / mol

Efficiencies of CaO/H2O/Ca(OH)2 chemical heat pump for heat storing and heating/cooling - ScienceDirect






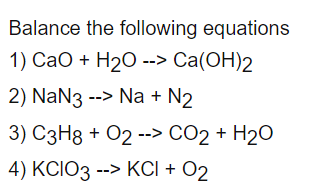




![Schematic diagram of CaO/Ca(OH) 2 TES [50-52]. | Download Scientific Diagram Schematic diagram of CaO/Ca(OH) 2 TES [50-52]. | Download Scientific Diagram](https://www.researchgate.net/publication/326700441/figure/fig1/AS:654390872518656@1533030350586/Schematic-diagram-of-CaO-CaOH-2-TES-50-52.png)