![Tandem Protocol of Hexahydroquinoline Synthesis Using [H2-DABCO][HSO4]2 Ionic Liquid as a Green Catalyst at Room Temperature | ACS Omega Tandem Protocol of Hexahydroquinoline Synthesis Using [H2-DABCO][HSO4]2 Ionic Liquid as a Green Catalyst at Room Temperature | ACS Omega](https://pubs.acs.org/cms/10.1021/acsomega.2c07672/asset/images/large/ao2c07672_0008.jpeg)
Tandem Protocol of Hexahydroquinoline Synthesis Using [H2-DABCO][HSO4]2 Ionic Liquid as a Green Catalyst at Room Temperature | ACS Omega

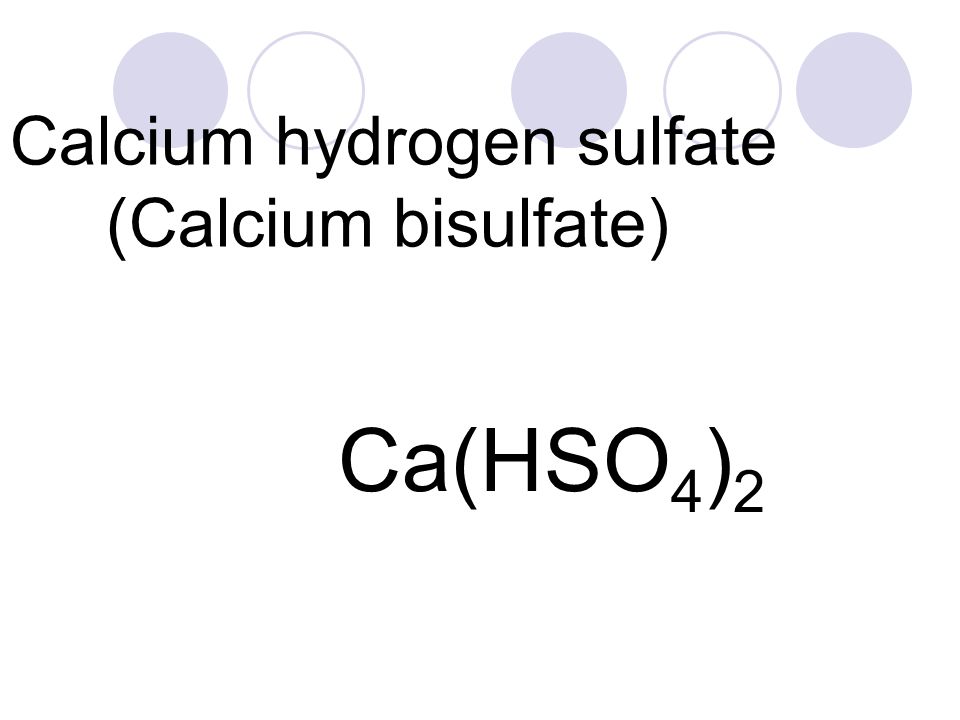
Из перечня формул выпишите отдельно формулы средних, кислых и основных солей, дайте их названия и запишите уравнения их диссоциации: Ca(HSO4)2, (CaOH)2SO4, Ca(NO3)2, NaH2PO4, Na3PO4, MgOHNO3.

Synthesis, characterization and temperature-triggered phase transition of organic-inorganic hybrid compound: (C6H18N2)(HSO4)2 - ScienceDirect
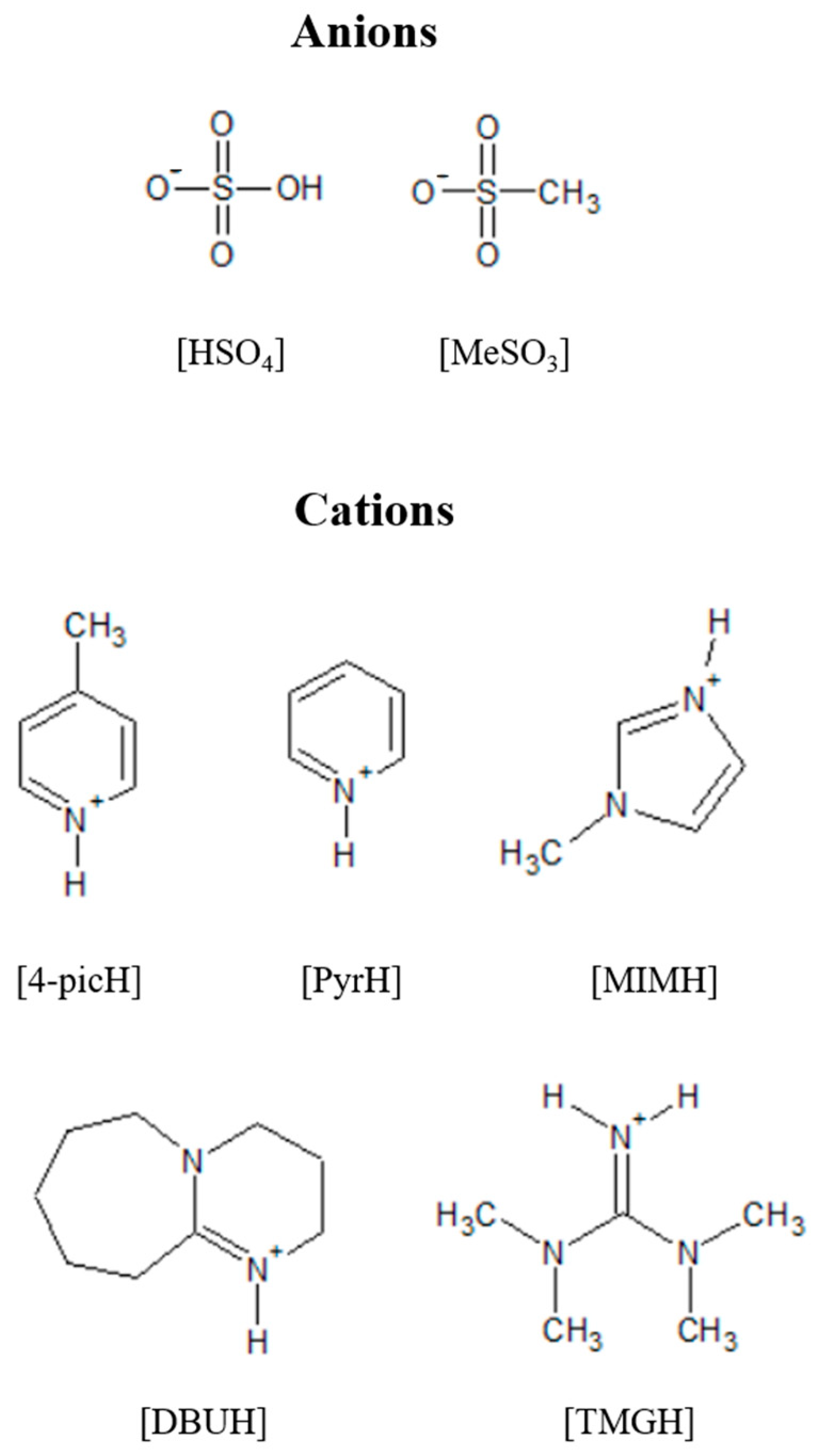
Molecules | Free Full-Text | New Protic Ionic Liquids as Potential Additives to Lubricate Si-Based MEMS/NEMS
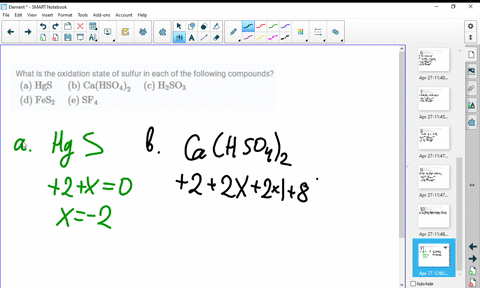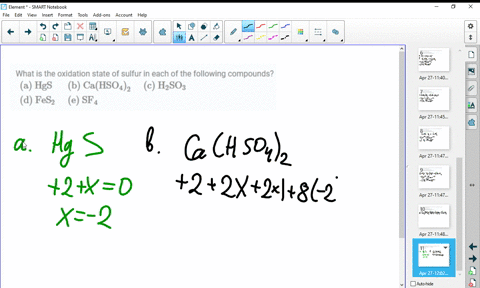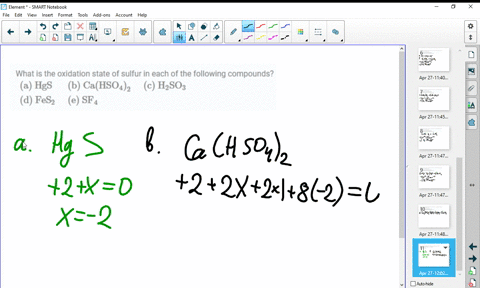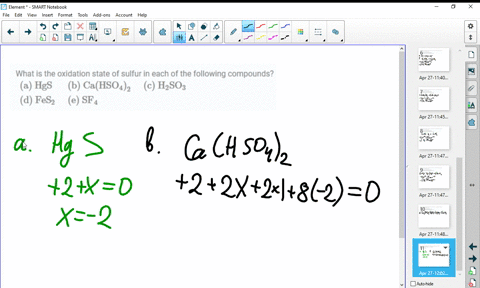
SOLVED:What is the oxidation state of sulfur in each of the following compounds? (a) HgS (b) Ca(HSO4)2 (c) H2SO3 (d) FeS2 (e) SF4
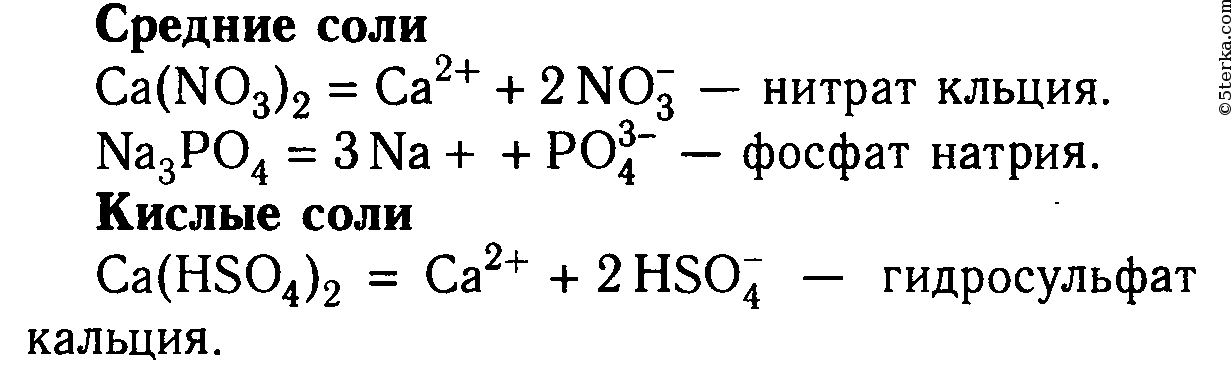
1 Из перечня формул выпишите отдельно формулы средних, кислых и основных солей, дайте их названия и запишите уравнения их диссоциации: Ca(HSO4)2, (CaOH)2SO4, Ca(NO3)2, NaH2PO4, Na3PO4, MgOHNO3.

PDF) Ca(HSO4)2 mediated conversion of alcohols into N-substituted amides under heterogeneous conditions: A modified Ritter reaction
![Tandem Protocol of Hexahydroquinoline Synthesis Using [H2-DABCO][HSO4]2 Ionic Liquid as a Green Catalyst at Room Temperature | ACS Omega Tandem Protocol of Hexahydroquinoline Synthesis Using [H2-DABCO][HSO4]2 Ionic Liquid as a Green Catalyst at Room Temperature | ACS Omega](https://pubs.acs.org/cms/10.1021/acsomega.2c07672/asset/images/acsomega.2c07672.social.jpeg_v03)
Tandem Protocol of Hexahydroquinoline Synthesis Using [H2-DABCO][HSO4]2 Ionic Liquid as a Green Catalyst at Room Temperature | ACS Omega
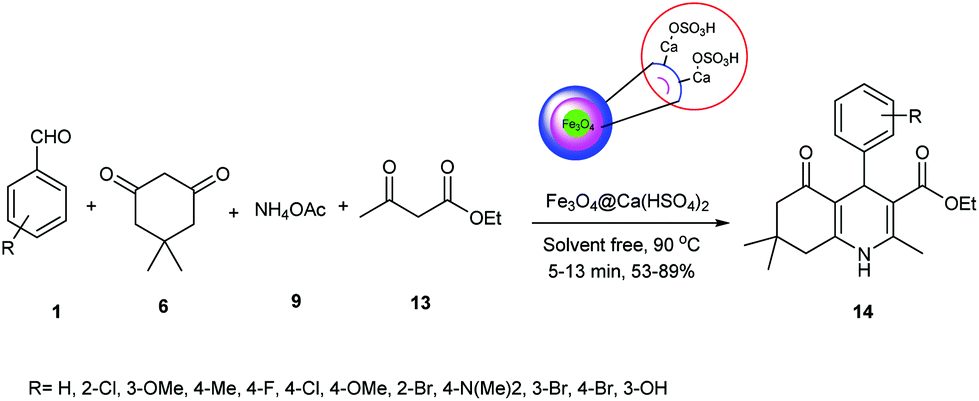
Waste-to-wealth transition: application of natural waste materials as sustainable catalysts in multicomponent reactions - Green Chemistry (RSC Publishing) DOI:10.1039/D2GC00704E

PDF) Ca(HSO4)2 mediated conversion of alcohols into N-substituted amides under heterogeneous conditions: A modified Ritter reaction